Global Craniofacial Implants Market - Key Trends & Drivers Summarized
Why Are Craniofacial Implants Gaining Global Significance in Reconstructive and Functional Restoration?
Craniofacial implants have become an essential component of modern reconstructive surgery, addressing both functional deficits and aesthetic restoration following trauma, congenital deformities, tumor resections, and craniosynostosis corrections. These implants support the repair or replacement of structural elements in the skull, jaw, orbit, and midface - enhancing quality of life by restoring normal anatomy, protecting intracranial contents, and enabling sensory and motor functionality.In trauma cases involving blunt force injury, ballistic trauma, or vehicular accidents, craniofacial implants provide critical stabilization and defect closure, often reducing neurological complications and infection risk. In oncology and neurosurgical domains, they facilitate reconstruction following tumor excision while allowing for cranial contour restoration and soft tissue support. Pediatric surgery is another key application area, where implants play a vital role in correcting syndromic craniofacial anomalies. With increased survival rates from complex injuries and greater patient expectations regarding post-surgical appearance, demand for high-precision, biocompatible craniofacial implants is growing globally.
What Technological Innovations Are Transforming Implant Design, Manufacturing, and Surgical Integration?
Additive manufacturing, patient-specific design, and biomaterial engineering are revolutionizing craniofacial implant technology. 3D printing is now widely used to create custom implants based on patient CT or MRI data, ensuring precise anatomical fit and minimizing intraoperative reshaping. These implants can be produced from a range of materials - including PEEK (polyetheretherketone), titanium, porous polyethylene, and bioresorbable polymers - each selected based on load-bearing requirements, biological integration, and radiolucency.Computer-aided design (CAD) and finite element modeling (FEM) are being applied to simulate mechanical stresses and optimize implant geometry for stability and osseointegration. Advances in surface coating technologies, such as hydroxyapatite and titanium plasma spray, enhance cell adhesion and long-term integration with host tissue. Some systems now include porous architecture or lattice structures to promote vascularization and osteoconductivity in large or complex defects.
In the surgical suite, intraoperative navigation systems, robotic-assisted placement, and augmented reality overlays are increasing procedural precision and reducing complication rates. Resorbable fixation systems are gaining popularity in pediatric and trauma cases, eliminating the need for secondary surgeries. Additionally, hybrid implant systems that combine soft-tissue prosthetics with cranial fixation are being introduced to address both skeletal and aesthetic rehabilitation in a single procedure.
Who Are the End-Users and How Are Clinical Practices and Regional Dynamics Driving Adoption?
Craniofacial implants are primarily used by neurosurgeons, maxillofacial surgeons, ENT specialists, trauma surgeons, and plastic/reconstructive surgeons operating in tertiary care centers, trauma hospitals, and academic institutions. High-volume users include craniofacial trauma centers, head and neck cancer units, and congenital anomaly correction teams. In pediatric applications, specialist children's hospitals collaborate with implant manufacturers to design growth-accommodating, bioresorbable implants for early-onset craniosynostosis and facial asymmetries.North America and Western Europe remain dominant markets due to advanced healthcare infrastructure, reimbursement coverage, and high rates of elective and corrective surgery. In the U.S., custom cranial implants are often covered under medical necessity clauses for trauma, deformity correction, and reconstructive procedures. In Europe, centralized healthcare systems support craniofacial surgeries within nationalized cranial reconstruction protocols. Asia-Pacific is witnessing rapid growth, fueled by medical tourism in countries like India, Thailand, and South Korea, as well as increasing access to neurosurgical care and aesthetic medicine in China and Southeast Asia.
Emerging markets in Latin America and the Middle East are expanding access to trauma and reconstructive services, creating new demand for affordable, semi-custom implant solutions. International NGOs and surgical outreach programs are also driving adoption in underserved regions, often through partnerships with craniofacial implant providers who donate or subsidize implant systems.
What Factors Are Powering Growth in the Craniofacial Implants Market Globally?
The growth in the craniofacial implants market is driven by rising incidences of head trauma, congenital anomalies, and cranial tumor surgeries, combined with advancements in surgical technique and biomaterials. Increasing availability of 3D planning tools and custom manufacturing workflows is making patient-specific reconstruction more accessible and reproducible. Simultaneously, a growing emphasis on functional and cosmetic outcomes is expanding surgical indications and patient volumes.Regulatory approvals and market clearances for novel materials and implant systems are accelerating commercialization timelines. Collaborations between hospitals, research institutes, and medtech companies are fostering innovation in implant design and surgical training. Government investments in neurotrauma centers and cleft-craniofacial programs are further widening access to surgical care.
The increasing integration of implants into multidisciplinary surgical workflows - spanning neurosurgery, plastic surgery, and oncology - ensures continued demand across both emergent and elective procedures. As personalized medicine, surgical robotics, and regenerative biomaterials converge, the craniofacial implants market is positioned for sustained, innovation-led growth that bridges clinical precision with patient-centric rehabilitation outcomes.
Report Scope
The report analyzes the Craniofacial Implants market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Material (Metal, Polymers, Other Materials); Product Type (Plate, Mesh, Paste, Screws); End-User (Hospitals, Plastic Surgery Clinics, Ambulatory Surgery Centers).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Metal Material segment, which is expected to reach US$58.1 Million by 2030 with a CAGR of a 8.6%. The Polymers Material segment is also set to grow at 6.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $16.5 Million in 2024, and China, forecasted to grow at an impressive 12.2% CAGR to reach $20.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Craniofacial Implants Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Craniofacial Implants Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Craniofacial Implants Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AIG Travel Guard, Allianz Care, Anvil Group, Assist America, AXA Assistance and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Craniofacial Implants market report include:
- Acumed LLC
- Alpha Aesthetics (AART, Inc.)
- Anatomics Pty Ltd
- B. Braun Melsungen AG
- Biocomposites Ltd.
- Bioplate Inc.
- Calavera Surgical Design
- Integra LifeSciences Holdings Corporation
- Johnson & Johnson (DePuy Synthes)
- KLS Martin SE & Co. KG
- Matrix Surgical USA
- Medartis AG
- Medtronic plc
- OssDsign AB
- OsteoMed
- Renishaw plc
- Stryker Corporation
- Xilloc Medical Int B.V.
- Zimmer Biomet Holdings, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Acumed LLC
- Alpha Aesthetics (AART, Inc.)
- Anatomics Pty Ltd
- B. Braun Melsungen AG
- Biocomposites Ltd.
- Bioplate Inc.
- Calavera Surgical Design
- Integra LifeSciences Holdings Corporation
- Johnson & Johnson (DePuy Synthes)
- KLS Martin SE & Co. KG
- Matrix Surgical USA
- Medartis AG
- Medtronic plc
- OssDsign AB
- OsteoMed
- Renishaw plc
- Stryker Corporation
- Xilloc Medical Int B.V.
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 383 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
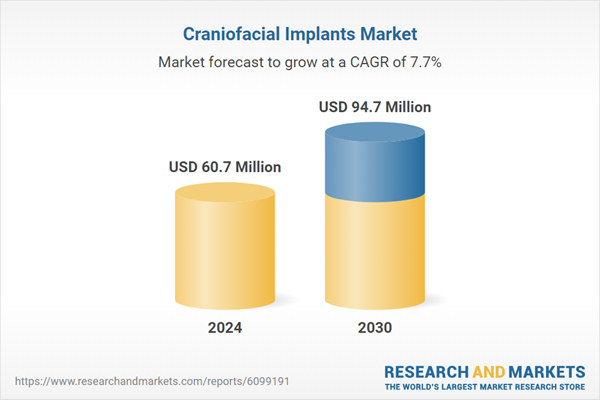
| Estimated Market Value ( USD | $ 60.7 Million |
| Forecasted Market Value ( USD | $ 94.7 Million |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |