Global Levothyroxine Market - Key Trends & Drivers Summarized
Why Does Levothyroxine Remain the Foundational Therapy in Thyroid Hormone Replacement?
Levothyroxine continues to dominate the treatment landscape for hypothyroidism due to its pharmacological efficacy, consistent clinical outcomes, and broad availability. As a synthetic form of the T4 hormone, levothyroxine is bioidentical to endogenous thyroxine and effectively normalizes thyroid-stimulating hormone (TSH) levels in patients with underactive thyroid glands. It is the first-line treatment recommended in clinical guidelines across the globe and is routinely prescribed for conditions such as Hashimoto's thyroiditis, post-thyroidectomy states, congenital hypothyroidism, and radioiodine-induced thyroid ablation. Its long half-life, typically around seven days, allows for once-daily dosing and stable serum hormone levels, contributing to high patient compliance and therapeutic continuity.The widespread prevalence of hypothyroidism, especially among women and the elderly, sustains demand for levothyroxine across both developed and emerging markets. The increasing incidence of autoimmune thyroid disorders and rising detection through routine screenings are further expanding the treatment population. Additionally, advancements in diagnostic tools such as ultrasensitive TSH assays and improved clinical awareness have led to earlier initiation of therapy. As a result, levothyroxine has become a chronic, lifelong therapy for a substantial proportion of patients, embedding it deeply into endocrinology care protocols and national essential drug lists.
How Are Formulation Innovations and Personalized Dosing Enhancing Market Relevance?
Despite being a mature therapy, the levothyroxine market is experiencing steady innovation in formulations designed to optimize absorption, stability, and patient adherence. Traditional tablet forms are now being complemented by soft gel capsules, liquid formulations, and chewable tablets, offering alternatives for patients with gastrointestinal absorption issues, dysphagia, or pediatric needs. Liquid levothyroxine, in particular, is gaining popularity among patients with malabsorption syndromes or those on medications that interfere with gastric pH, such as proton pump inhibitors. These innovations cater to a growing demand for more individualized and tolerable treatment options.Personalized dosing is also becoming a focal point, especially with the recognition that small deviations in serum thyroxine can lead to significant symptomatic differences. Weight-based, age-adjusted, and comorbidity-informed titration protocols are increasingly utilized in clinical practice. Pharmacogenomic insights - particularly involving deiodinase polymorphisms and thyroid hormone transporter genes - are beginning to influence dosing strategies in select patient populations. Furthermore, digital health tools, including dosing calculators, medication trackers, and telehealth consults, are improving real-time therapy adjustments and supporting more responsive patient management. These dynamics are reinforcing levothyroxine's relevance while mitigating the clinical inertia often associated with legacy pharmaceuticals.
What Are the Regulatory, Quality, and Supply Chain Complexities in the Levothyroxine Market?
Levothyroxine is uniquely sensitive to manufacturing consistency and bioavailability variations, which has resulted in strict regulatory oversight across major health authorities. Even small differences in formulation between branded and generic versions can lead to clinically significant changes in TSH levels, necessitating narrow therapeutic index (NTI) classification in several jurisdictions. As a result, regulators such as the FDA, EMA, and TGA impose stringent bioequivalence requirements and mandate stability testing across a range of storage conditions. These regulations have also prompted recommendations that patients consistently use the same brand or manufacturer to avoid therapeutic variability.From a supply chain perspective, levothyroxine's global availability depends on complex manufacturing and distribution ecosystems. Raw material sourcing, especially for active pharmaceutical ingredients (APIs), is concentrated in select regions, primarily India and China. Disruptions in these supply lines due to geopolitical tensions or regulatory non-compliance can lead to periodic shortages, as observed in several markets over the past decade. Furthermore, pricing controls in public health systems often affect the profitability of levothyroxine products, discouraging new entrants and contributing to market consolidation. This creates pressure points that governments are increasingly seeking to address through strategic stockpiling, local manufacturing incentives, and tighter quality surveillance.
What Is Propelling Growth and Sustained Demand in the Levothyroxine Market?
The growth in the levothyroxine market is driven by several factors including rising hypothyroidism prevalence, demographic aging, and improved access to diagnostic and therapeutic care. Increased awareness about thyroid disorders among primary care physicians, gynecologists, and general practitioners is leading to more frequent testing and earlier diagnosis. This, in turn, drives long-term prescriptions of levothyroxine, especially in regions with aging populations where thyroid dysfunction is more common. Epidemiological data also points to a growing burden of subclinical hypothyroidism and thyroid hormone resistance syndromes, expanding the treatment base beyond overtly symptomatic cases.Pharmaceutical strategies around lifecycle management and geographic expansion are further contributing to market momentum. Manufacturers are launching novel delivery systems, flavor-masked pediatric formulations, and patient-friendly packaging to differentiate their products. Emerging economies in Asia, Latin America, and Africa are witnessing improved access to thyroid diagnostics and therapies through national health programs, driving greater levothyroxine uptake. The increasing use of e-pharmacies and chronic medication delivery platforms is also facilitating better therapy continuity, particularly in urbanizing regions with limited access to endocrinologists.
Additionally, the medical community's growing interest in optimizing thyroid levels for fertility outcomes, cardiovascular health, and mental well-being is strengthening the case for proactive levothyroxine use. Clinical trials exploring its use in euthyroid individuals with elevated TSH in pregnancy or those with depression symptoms are expanding potential therapeutic boundaries. These converging trends are ensuring that levothyroxine remains not just a volume-driven product, but a clinically relevant and strategically evolving component of endocrine care worldwide.
Report Scope
The report analyzes the Levothyroxine market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Administration Route (Oral, Injectables); Distribution Channel (Hospital Pharmacies Distribution Channel, Retail Pharmacies Distribution Channel, Online Pharmacies Distribution Channel).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Oral Administration segment, which is expected to reach US$2.9 Billion by 2030 with a CAGR of a 2.9%. The Injectables Administration segment is also set to grow at 1.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1 Billion in 2024, and China, forecasted to grow at an impressive 4.7% CAGR to reach $843.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Levothyroxine Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Levothyroxine Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Levothyroxine Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie Inc., Acorda Therapeutics, Inc., Amneal Pharmaceuticals, Inc., Bristol-Myers Squibb Co., Eli Lilly and Company and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Levothyroxine market report include:
- Abbott Laboratories
- AbbVie Inc.
- Advanz Pharma Corp.
- Aurobindo Pharma Ltd.
- Cipla Ltd.
- Dr. Reddy's Laboratories Ltd.
- Fresenius Kabi AG
- GlaxoSmithKline plc
- IBSA Institut Biochimique SA
- Lannett Company, Inc.
- Lupin Limited
- Merck & Co., Inc.
- Mylan N.V. (Viatris Inc.)
- Novartis AG
- Pfizer Inc.
- Piramal Enterprises Ltd.
- Sandoz International GmbH
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Zydus Lifesciences Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- AbbVie Inc.
- Advanz Pharma Corp.
- Aurobindo Pharma Ltd.
- Cipla Ltd.
- Dr. Reddy's Laboratories Ltd.
- Fresenius Kabi AG
- GlaxoSmithKline plc
- IBSA Institut Biochimique SA
- Lannett Company, Inc.
- Lupin Limited
- Merck & Co., Inc.
- Mylan N.V. (Viatris Inc.)
- Novartis AG
- Pfizer Inc.
- Piramal Enterprises Ltd.
- Sandoz International GmbH
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Zydus Lifesciences Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 268 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
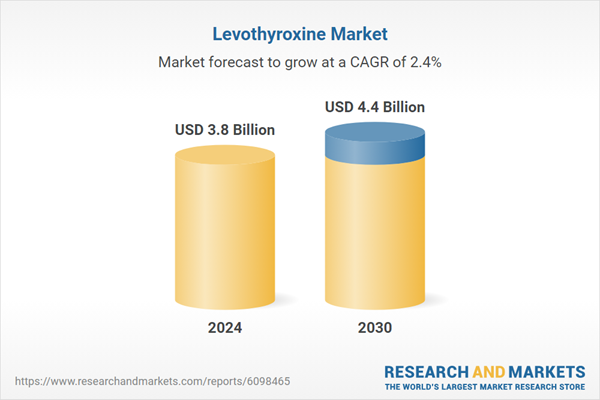
| Estimated Market Value ( USD | $ 3.8 Billion |
| Forecasted Market Value ( USD | $ 4.4 Billion |
| Compound Annual Growth Rate | 2.4% |
| Regions Covered | Global |