Global Obesity Intervention Devices Market - Key Trends & Drivers Summarized
Why Are Obesity Intervention Devices Gaining Clinical Ground Amid a Global Obesity Epidemic?
Obesity intervention devices are becoming an increasingly important element in the treatment of moderate-to-severe obesity, particularly among individuals for whom lifestyle changes and pharmacotherapy have failed. With over 1 billion people globally classified as obese, the health implications - ranging from type 2 diabetes and cardiovascular disease to obstructive sleep apnea and musculoskeletal disorders - are driving urgent demand for durable, safe, and minimally invasive solutions. Obesity intervention devices offer alternatives to traditional bariatric surgery, with lower procedural risks and shorter recovery times, making them more accessible to a wider patient demographic.The market includes intragastric balloons, endoscopic sleeve gastroplasty (ESG) devices, vagal nerve stimulation implants, duodenal-jejunal bypass liners, and gastric electrical stimulators. These devices operate via mechanisms such as gastric volume reduction, delayed gastric emptying, appetite suppression, or hormonal modulation to achieve clinically significant weight loss. The rising adoption of these devices is fueled by the growing clinical validation of their efficacy, increasing insurance coverage, and patient preference for non-permanent, reversible interventions. As obesity continues to drive global morbidity and healthcare expenditure, device-based solutions are gaining momentum in integrated weight management strategies.
How Are Innovation and Minimally Invasive Approaches Transforming Device-Based Obesity Treatment?
Recent advancements in device design, material science, and endoscopic delivery systems are transforming obesity intervention into a field of precision, minimally invasive therapeutics. New-generation intragastric balloons use biodegradable materials, adjustable volume features, and self-deflating safety mechanisms, enhancing both efficacy and patient safety. Endoluminal devices delivered via flexible endoscopes are reducing reliance on laparoscopic surgery and general anesthesia, making procedures more suitable for outpatient care or ambulatory surgical centers.Gastric electrical stimulation technologies are evolving with closed-loop feedback systems and machine learning algorithms that tailor neuromodulation based on satiety signals or gastric motility patterns. Robotic assistance, 3D imaging, and digital integration are improving procedural accuracy and post-procedure follow-up. These technological advances are not only expanding the market size by lowering the barrier to entry for patients but also reducing complication rates, minimizing recovery times, and improving long-term outcomes. As the field evolves, the integration of smart, adaptive, and reversible devices is defining the next generation of obesity treatment.
Which Patient Demographics and Regional Markets Are Driving the Uptake of Obesity Intervention Devices?
Obesity intervention devices are primarily targeted at adults with body mass index (BMI) values between 30 and 40 kg/m², especially those with obesity-related comorbidities or prior pharmacotherapy failures. Younger patients seeking weight loss solutions that are less invasive than surgery and older patients ineligible for general anesthesia represent two fast-growing demographic segments. Devices are also being explored for adolescents with severe obesity under controlled trials, reflecting a broader clinical recognition of early intervention.North America leads global adoption, with the U.S. accounting for a significant share due to high obesity prevalence, FDA approvals for novel devices, and reimbursement growth under private and public insurance. Europe follows, with strong uptake in countries like Germany, Spain, and the U.K. where endoscopic bariatric therapies are integrated into national health systems. Asia-Pacific is witnessing rapid expansion driven by urbanization-linked obesity trends, rising healthcare access, and growing demand for outpatient weight loss solutions. The Middle East, Latin America, and parts of Africa are increasingly adopting devices through medical tourism, subsidized public health programs, and lifestyle clinics.
What Is Fueling Long-Term Growth and Strategic Innovation in the Obesity Intervention Devices Market?
The growth in the obesity intervention devices market is driven by a confluence of rising obesity rates, unmet therapeutic needs, and the demand for non-pharmacological, minimally invasive weight loss alternatives. Regulatory approvals for new device classes, improved reimbursement frameworks, and long-term clinical evidence of weight loss maintenance and metabolic benefit are boosting confidence among clinicians and patients. Public health agencies are increasingly recognizing device-based therapy as part of multi-modal obesity management guidelines.Ongoing research into gut-brain axis modulation, metabolic surgery analogues, and hormonal feedback manipulation is likely to yield next-generation devices with enhanced metabolic effects. Partnerships between device manufacturers, telehealth providers, and behavioral coaching platforms are improving adherence and enabling long-term lifestyle change. As weight loss becomes a gateway to broader metabolic health, obesity intervention devices will continue to play a transformative role in preventive medicine, cardiometabolic risk management, and global public health strategy.
Report Scope
The report analyzes the Obesity Intervention Devices market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Device (Gastric Bands, Gastric Balloon, Gastric Stimulation System); End-User (Hospitals, Clinics).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Gastric Bands segment, which is expected to reach US$177.2 Million by 2030 with a CAGR of a 2.9%. The Gastric Balloon segment is also set to grow at 4.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $75.1 Million in 2024, and China, forecasted to grow at an impressive 6.6% CAGR to reach $67.9 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Obesity Intervention Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Obesity Intervention Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Obesity Intervention Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AgriProtein Holdings Ltd., AgriReNew, Inc., Algol Chemicals, Anuvia Plant Nutrients, Aqua Green and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Obesity Intervention Devices market report include:
- A.M.I. GmbH
- AbbVie Inc.
- Allurion Technologies, Inc.
- Apollo Endosurgery, Inc.
- Aspire Bariatrics, Inc.
- BAROnova, Inc.
- Boston Scientific Corporation
- Cousin Surgery
- Endalis Laboratoire
- Gelesis, Inc.
- GI Dynamics, Inc.
- Intuitive Surgical, Inc.
- Johnson & Johnson (Ethicon)
- Mediflex Surgical Products
- Medtronic plc
- Olympus Corporation
- ReShape Lifesciences, Inc.
- Spatz FGIA, Inc.
- Stryker Corporation
- TransEnterix Surgical, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- A.M.I. GmbH
- AbbVie Inc.
- Allurion Technologies, Inc.
- Apollo Endosurgery, Inc.
- Aspire Bariatrics, Inc.
- BAROnova, Inc.
- Boston Scientific Corporation
- Cousin Surgery
- Endalis Laboratoire
- Gelesis, Inc.
- GI Dynamics, Inc.
- Intuitive Surgical, Inc.
- Johnson & Johnson (Ethicon)
- Mediflex Surgical Products
- Medtronic plc
- Olympus Corporation
- ReShape Lifesciences, Inc.
- Spatz FGIA, Inc.
- Stryker Corporation
- TransEnterix Surgical, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 278 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
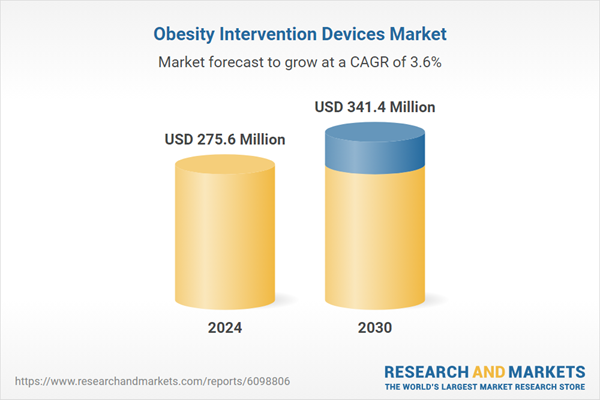
| Estimated Market Value ( USD | $ 275.6 Million |
| Forecasted Market Value ( USD | $ 341.4 Million |
| Compound Annual Growth Rate | 3.6% |
| Regions Covered | Global |