Global Ocular Drug Delivery Market - Key Trends & Drivers Summarized
Why Is Ocular Drug Delivery Undergoing a Paradigm Shift in Treating Vision-Threatening Disorders?
Ocular drug delivery is a rapidly evolving domain in ophthalmology, driven by the need to deliver therapeutic agents directly to target tissues within the eye while overcoming anatomical barriers and maintaining sustained therapeutic concentrations. The unique structure of the eye - including protective barriers like the cornea, blood-aqueous barrier, and blood-retinal barrier - makes conventional systemic or topical drug delivery largely ineffective for treating posterior segment diseases. This has necessitated innovation in localized, targeted, and extended-release drug delivery platforms.The rising global burden of ocular diseases such as age-related macular degeneration (AMD), diabetic retinopathy, glaucoma, and uveitis is creating unprecedented demand for effective and patient-compliant delivery systems. Traditional routes such as eye drops and intravitreal injections are limited by poor bioavailability, short duration of action, and patient adherence issues. Emerging technologies, including implants, nano-carriers, in situ forming gels, and microneedle arrays, are addressing these limitations by enhancing drug penetration, extending release profiles, and minimizing invasiveness. As sight-threatening conditions become more prevalent with aging populations and rising diabetes rates, ocular drug delivery is becoming a critical pillar of ophthalmic care.
How Are Drug Delivery Innovations Improving Efficacy, Safety, and Patient Compliance in Ophthalmology?
Cutting-edge ocular drug delivery platforms are achieving longer therapeutic duration and tissue specificity through a range of technologies, including biodegradable implants (e.g., dexamethasone intravitreal implants), refillable reservoirs, and nanoparticle-based carriers. These systems release drugs over weeks to months, reducing the frequency of intravitreal injections and thereby improving patient compliance and clinical outcomes. Sustained-release corticosteroids and anti-VEGF agents are now routinely used in managing chronic retinal diseases with fewer interventions.Topical nanomicelles, liposomes, dendrimers, and hydrogels are being developed to enable anterior and posterior segment delivery without the need for injections. In situ-forming gels and ocular inserts that respond to temperature, pH, or enzymatic triggers are improving drug residence time and therapeutic response. Implantable micro-pumps and electronic devices capable of controlled drug release are also in development, representing the convergence of digital therapeutics with pharmaceutical delivery. These technologies are redefining the pharmacokinetic and pharmacodynamic landscape of ocular therapeutics, enabling precise, programmable, and patient-friendly treatment paradigms.
Which Clinical Indications and Regional Healthcare Systems Are Driving Demand for Advanced Ocular Delivery Solutions?
Chronic posterior segment disorders - such as AMD, diabetic macular edema (DME), and retinal vein occlusion - are the largest drivers of demand for intravitreal and sustained-release drug delivery systems. Glaucoma treatment is also shifting toward implantable devices and controlled-release mechanisms to improve adherence and intraocular pressure control. Pediatric and geriatric ophthalmology are additional growth areas where drug delivery challenges and compliance issues are particularly acute, highlighting the need for once-daily or depot formulations.North America and Europe lead in terms of regulatory approvals, commercial product availability, and specialist ophthalmic care infrastructure. The U.S. market has witnessed rapid adoption of FDA-approved implants and nanoformulations, while the European market benefits from centralized reimbursement and high unmet need. Asia-Pacific is witnessing high demand growth, particularly in Japan, India, and China, due to aging populations, diabetic demographics, and government-led blindness prevention initiatives. Latin America and the Middle East are expanding access through public-private partnerships and eye-care NGO programs that increasingly incorporate advanced drug delivery platforms.
What Is Driving Long-Term Growth and Cross-Disciplinary Innovation in the Ocular Drug Delivery Market?
The growth in the ocular drug delivery market is driven by rising vision loss prevalence, treatment burden reduction imperatives, and advances in pharmaceutical engineering, biomaterials, and nanotechnology. As pharmaceutical companies race to differentiate ophthalmic portfolios through patient-centric innovations, collaboration between drug developers, device manufacturers, and materials scientists is yielding transformative therapies. Biosimilars, gene therapies, and dual-drug platforms are being integrated with delivery systems that optimize ocular bioavailability while minimizing side effects.Artificial intelligence, remote monitoring, and smart ophthalmic devices are being explored to complement drug delivery and track therapeutic response. Regulatory incentives for orphan ophthalmic drugs and fast-track designations for innovative delivery platforms are accelerating market entry. As the boundaries between pharmaceuticals, biologics, and devices blur, ocular drug delivery is becoming a focal point of precision medicine in ophthalmology - promising sight preservation, procedural simplicity, and life-changing outcomes for millions affected by chronic eye conditions.
Report Scope
The report analyzes the Ocular Drug Delivery market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Technology (Topical, Ocular Insert, Intraocular Implants, In-Situ Gel, Other Technologies); Formulation Type (Ointment, Solution, Emulsion, Suspension, Other Formulation Types); Disease Indication (Glaucoma, Diabetic Retinopathy, Dry Eye Syndrome, Age Related Macular Degeneration, Other Disease Indications); End-User (Hospitals, Ophthalmic Clinics, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Topical Technology segment, which is expected to reach US$35.2 Billion by 2030 with a CAGR of a 5.8%. The Ocular Insert Technology segment is also set to grow at 3.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $19.9 Billion in 2024, and China, forecasted to grow at an impressive 8.2% CAGR to reach $19.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Ocular Drug Delivery Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Ocular Drug Delivery Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Ocular Drug Delivery Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as A.M.I. GmbH, AbbVie Inc., Allurion Technologies, Inc., Apollo Endosurgery, Inc., Aspire Bariatrics, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Ocular Drug Delivery market report include:
- AbbVie Inc.
- Aciont Inc.
- Alcon
- Alimera Sciences, Inc.
- Allergan (an AbbVie company)
- Bausch Health Companies Inc.
- Clearside Biomedical, Inc.
- EyeGate Pharmaceuticals, Inc.
- Eyenovia, Inc.
- EyePoint Pharmaceuticals, Inc.
- Genentech, Inc.
- Graybug Vision, Inc.
- Iris Pharma
- Johnson & Johnson Vision Care, Inc.
- Kiora Pharmaceuticals, Inc.
- Nicox S.A.
- Novaliq GmbH
- Novartis AG
- Ocular Therapeutix, Inc.
- Oculis SA
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Aciont Inc.
- Alcon
- Alimera Sciences, Inc.
- Allergan (an AbbVie company)
- Bausch Health Companies Inc.
- Clearside Biomedical, Inc.
- EyeGate Pharmaceuticals, Inc.
- Eyenovia, Inc.
- EyePoint Pharmaceuticals, Inc.
- Genentech, Inc.
- Graybug Vision, Inc.
- Iris Pharma
- Johnson & Johnson Vision Care, Inc.
- Kiora Pharmaceuticals, Inc.
- Nicox S.A.
- Novaliq GmbH
- Novartis AG
- Ocular Therapeutix, Inc.
- Oculis SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 489 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
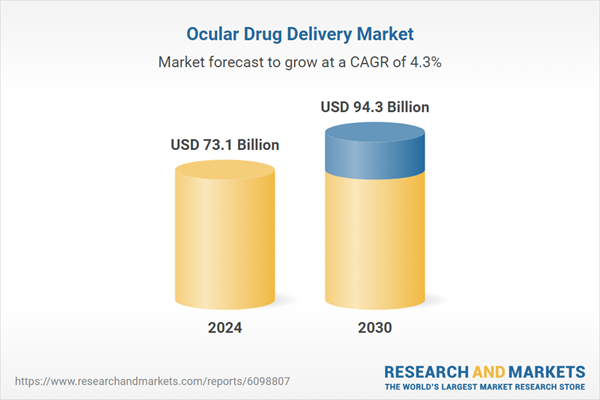
| Estimated Market Value ( USD | $ 73.1 Billion |
| Forecasted Market Value ( USD | $ 94.3 Billion |
| Compound Annual Growth Rate | 4.3% |
| Regions Covered | Global |