Global Plexiform Neurofibromas Treatment Market - Key Trends & Drivers Summarized
Why Is Plexiform Neurofibromas Treatment Gaining Urgency in Rare Tumor Management and Genetic Disorder Care?
Plexiform neurofibromas (PNs) are benign but often debilitating tumors associated with Neurofibromatosis Type 1 (NF1), a genetic disorder caused by mutations in the NF1 gene. These tumors can develop along peripheral nerves and infiltrate surrounding tissues, leading to pain, disfigurement, mobility limitations, and potentially life-threatening complications such as airway obstruction or malignant transformation. Treatment for PNs has historically been limited to surgical excision, which carries high recurrence rates and risks of nerve damage due to the tumor's diffuse and invasive nature.The introduction of targeted therapies - most notably MEK inhibitors such as selumetinib - has marked a paradigm shift in PN management. Approved by the FDA for pediatric patients with symptomatic, inoperable PNs, selumetinib inhibits the MAPK pathway downstream of the NF1 mutation, leading to tumor volume reduction and symptomatic improvement. This is the first pharmacologic treatment with demonstrated efficacy in shrinking PNs, offering new hope for patients previously reliant solely on surgery or supportive care. As clinical understanding of NF1 deepens, therapeutic development for PNs is accelerating within the broader field of neuro-oncology and genetic medicine.
How Are Drug Development and Gene-Targeted Therapies Expanding Treatment Options for NF1-Related Tumors?
Clinical pipelines are expanding with new MEK inhibitors (e.g., trametinib, binimetinib), mTOR inhibitors, and combination regimens designed to improve efficacy and address resistance mechanisms. Trials are underway to assess their safety and effectiveness in adult PN patients, as well as in combination with immunotherapies or radiologic interventions. Ongoing biomarker studies aim to identify responders and optimize dosage for maximal tumor control with minimal toxicity.In parallel, gene-editing and antisense oligonucleotide (ASO) therapies targeting the NF1 gene or its downstream effectors are entering preclinical development. These approaches seek to correct the underlying genetic dysfunction rather than merely inhibit proliferative signaling. Additionally, patient-derived xenografts and organoid models are being used to simulate PN behavior and evaluate new compounds in a personalized medicine framework. Advanced imaging modalities like volumetric MRI are also enhancing treatment monitoring by enabling precise measurement of tumor burden and response over time.
Which Patient Populations and Research Institutions Are Driving Therapeutic Demand and Innovation Globally?
Pediatric NF1 patients represent the largest initial treatment cohort, especially those with symptomatic, inoperable PNs that compromise function, cause pain, or distort anatomy. Adults with progressive PNs or those with a high risk of malignant peripheral nerve sheath tumor (MPNST) transformation are emerging as a secondary target population for therapeutic intervention. Multidisciplinary centers for neurofibromatosis, pediatric oncology hospitals, and clinical research organizations are leading patient recruitment, treatment trials, and data collection.North America - especially the U.S. - remains the epicenter of NF1 research and drug approval activity, supported by institutions such as the NIH, Children's Tumor Foundation, and leading university hospitals. Europe is also active, with research hubs in Germany, France, and the UK conducting collaborative trials through consortia like the European Reference Network for Rare Neurological Diseases. Asia-Pacific is increasingly involved in observational studies and early-phase trials, with growing awareness and diagnosis rates supporting demand. As genetic screening becomes more routine, global identification and management of PN patients is expected to expand significantly.
What Is Driving Long-Term Growth and Strategic Advancement in the Plexiform Neurofibromas Treatment Market?
The growth in the plexiform neurofibromas treatment market is driven by advances in genetic research, orphan drug policy incentives, and increased advocacy for rare disease therapies. As regulators offer expedited pathways for breakthrough treatments, pharmaceutical companies are investing in precision drugs that meet unmet clinical needs in NF1. Orphan drug designations, market exclusivity, and pediatric priority review vouchers are fueling innovation and reducing time-to-market for effective PN therapies.Strategically, biopharma firms are forming partnerships with academic centers, patient advocacy organizations, and genomic data platforms to develop and commercialize therapies. Companion diagnostics, biomarker-driven trials, and real-world evidence registries are enhancing understanding of disease progression and treatment response. As awareness of NF1 grows within both the clinical and patient communities, early diagnosis and intervention are becoming priorities - fueling demand for novel therapies that offer meaningful tumor control, improved quality of life, and potential long-term remission. In this emerging therapeutic frontier, PNs represent not only a medical challenge but a gateway to innovation in treating complex genetic disorders.
Report Scope
The report analyzes the Plexiform Neurofibromas Treatment market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Drug Class (Selumetinib, Non-Steroidal Anti-Inflammatory Drugs, Anticonvulsants, Other Drug Classes); Demographic (Pediatric, Adult); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Selumetinib segment, which is expected to reach US$632.4 Million by 2030 with a CAGR of a 5%. The Non-Steroidal Anti-Inflammatory Drugs segment is also set to grow at 8.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $335 Million in 2024, and China, forecasted to grow at an impressive 10% CAGR to reach $365.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Plexiform Neurofibromas Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Plexiform Neurofibromas Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Plexiform Neurofibromas Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Adobe Inc., Apple Inc., Canon Inc., Cubert GmbH, d'Optron Co., Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 39 companies featured in this Plexiform Neurofibromas Treatment market report include:
- AbbVie Inc.
- Alexion Pharmaceuticals, Inc.
- Amgen Inc.
- AstraZeneca plc
- Bayer AG
- BioMarin Pharmaceutical Inc.
- Bristol Myers Squibb
- CureAge Therapeutics
- Eli Lilly and Company
- Genentech, Inc.
- GlaxoSmithKline plc
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Regeneron Pharmaceuticals
- Roche Holding AG
- Sanofi S.A.
- SpringWorks Therapeutics, Inc.
- Takeda Pharmaceutical Company
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Alexion Pharmaceuticals, Inc.
- Amgen Inc.
- AstraZeneca plc
- Bayer AG
- BioMarin Pharmaceutical Inc.
- Bristol Myers Squibb
- CureAge Therapeutics
- Eli Lilly and Company
- Genentech, Inc.
- GlaxoSmithKline plc
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Regeneron Pharmaceuticals
- Roche Holding AG
- Sanofi S.A.
- SpringWorks Therapeutics, Inc.
- Takeda Pharmaceutical Company
Table Information
| Report Attribute | Details |
|---|---|
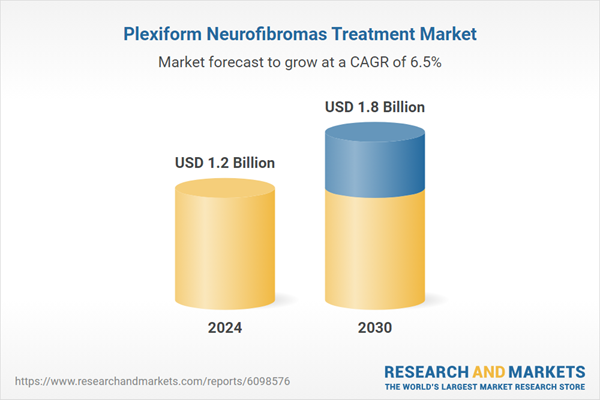
| No. of Pages | 372 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 1.2 Billion |
| Forecasted Market Value ( USD | $ 1.8 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |