Global Polio Vaccines Market - Key Trends & Drivers Summarized
Why Do Polio Vaccines Remain a Cornerstone of Global Public Health Despite Near-Eradication?
Polio vaccines have been the foundation of one of the most ambitious global public health campaigns ever undertaken - aiming to eliminate poliomyelitis, a crippling and potentially fatal infectious disease caused by the poliovirus. Despite significant progress, with wild poliovirus now endemic in only a few countries, continued vaccination remains critical due to risks of re-emergence, vaccine-derived poliovirus strains, and global mobility. Two primary vaccine types - oral polio vaccine (OPV) and inactivated polio vaccine (IPV) - remain central to immunization strategies in different regions depending on transmission risk, healthcare infrastructure, and regulatory frameworks.OPV, particularly the bivalent (bOPV) and novel oral polio vaccine type 2 (nOPV2), is widely used in mass immunization campaigns due to its ease of administration and ability to confer community protection through gut immunity and viral shedding. IPV, on the other hand, is preferred in high-income countries and in routine immunization schedules for its safety profile and lack of risk for vaccine-derived strains. As the global health community transitions to a polio endgame strategy, the co-administration of IPV and nOPV2 is being prioritized to ensure immunity, prevent outbreaks, and achieve long-term eradication.
How Are Technological Advancements and Strategic Formulations Enhancing Vaccine Efficacy and Safety?
Vaccine R&D has evolved to develop safer and more stable polio vaccines with improved immunogenicity and a lower risk of circulating vaccine-derived poliovirus (cVDPV). The introduction of nOPV2, engineered with genetically stabilized attenuation profiles, represents a breakthrough in addressing the challenges posed by type 2 poliovirus outbreaks linked to earlier OPV formulations. This vaccine is being deployed under WHO's Emergency Use Listing and has shown promising early field results in limiting the emergence of neurovirulent revertants.Advances in cold chain logistics, freeze-drying, and prefilled injectable formats have further expanded the reach and shelf life of polio vaccines, particularly in remote or conflict-affected regions. Combination vaccines incorporating IPV with diphtheria, pertussis, and tetanus (DPT-IPV) are gaining traction in routine immunization programs for their efficiency and broader coverage. The use of digital immunization registries, barcoded vials, and geospatial mapping in immunization campaigns is also improving traceability, monitoring, and data-driven deployment, thereby enhancing vaccination efficacy and accountability.
Which Global Alliances and Regional Programs Are Driving Polio Vaccine Distribution and Coverage?
The Global Polio Eradication Initiative (GPEI) - led by WHO, UNICEF, the U.S. CDC, Rotary International, and the Bill & Melinda Gates Foundation - has been the primary driver of vaccine funding, coordination, and field deployment for over three decades. National immunization days (NIDs), door-to-door campaigns, and outbreak response immunization efforts (ORIs) have all played pivotal roles in raising global polio vaccine coverage. Countries with weak health infrastructure or conflict zones remain priority areas, requiring targeted campaigns and cross-border coordination.South Asia and sub-Saharan Africa have historically been focal points for vaccine delivery, although most regions have now transitioned from trivalent OPV (tOPV) to bOPV and IPV. Afghanistan and Pakistan remain the last strongholds of wild poliovirus, necessitating ongoing surveillance and high-frequency immunization drives. In developed regions such as North America and Europe, IPV is standard in national immunization schedules, with focus now shifting to bolstering preparedness and maintaining herd immunity. As global eradication inches closer, regional surveillance and sustained vaccine uptake will be essential to prevent resurgence.
What Is Driving Long-Term Strategic Focus and Innovation in the Polio Vaccines Market?
The growth in the polio vaccines market is driven by ongoing global eradication efforts, emergence of cVDPV outbreaks, and international health security mandates. While eradication may ultimately reduce the size of the routine polio vaccine market, short- to medium-term demand is expected to remain high due to emergency responses, transition plans, and stockpile requirements. Governments and NGOs are committed to maintaining high coverage and vaccine stockpiles to ensure polio's irreversible elimination.Strategically, manufacturers are investing in next-generation IPV production using Vero cell and Sabin strain platforms to increase biosafety and reduce production costs. Technology transfers and local manufacturing initiatives - especially in India, Indonesia, and parts of Africa - are enhancing regional supply chain resilience. Public-private partnerships, pooled procurement by UNICEF and Gavi, and continued innovation in vaccine packaging and delivery are reinforcing the global vaccination infrastructure. As the polio endgame approaches, the vaccine market will pivot from volume-based supply to a strategic reserve-driven, precision-deployment model aimed at complete and sustained eradication.
Report Scope
The report analyzes the Polio Vaccines market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Inactivated Polio Vaccine, Oral Polio Vaccine); End-Use (Hospitals & Clinics End-Use, Public Services End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Inactivated Polio Vaccine segment, which is expected to reach US$624 Million by 2030 with a CAGR of a 2.6%. The Oral Polio Vaccine segment is also set to grow at 4.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $220.4 Million in 2024, and China, forecasted to grow at an impressive 5.9% CAGR to reach $192.1 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Polio Vaccines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Polio Vaccines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Polio Vaccines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AAE (Aluminum Athletic Equipment), Altius Poles, Aluminum Athletic Equipment Co., ARH Sports Equipment Ltd., Benz Sport and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Polio Vaccines market report include:
- Adithya Vaccine Pharma
- Astellas Pharma Inc.
- Beijing Tiantan Biological Products Corp
- Bharat Biotech International Ltd.
- Bharat Immunologicals and Biologicals Corporation Limited (BIBCOL)
- Bio Farma
- Bio-Med Healthcare Products Pvt. Ltd.
- Biomed-Lublin
- Boehringer Ingelheim International GmbH
- F. Hoffmann-La Roche Ltd.
- GlaxoSmithKline (GSK)
- Haffkine Bio-Pharmaceutical Corporation Ltd.
- Merck & Co., Inc.
- Mylan N.V.
- Novartis AG
- Panacea Biotec Ltd.
- Pfizer Inc.
- Sanofi Pasteur
- Serum Institute of India Pvt. Ltd.
- Sinovac Biotech Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Adithya Vaccine Pharma
- Astellas Pharma Inc.
- Beijing Tiantan Biological Products Corp
- Bharat Biotech International Ltd.
- Bharat Immunologicals and Biologicals Corporation Limited (BIBCOL)
- Bio Farma
- Bio-Med Healthcare Products Pvt. Ltd.
- Biomed-Lublin
- Boehringer Ingelheim International GmbH
- F. Hoffmann-La Roche Ltd.
- GlaxoSmithKline (GSK)
- Haffkine Bio-Pharmaceutical Corporation Ltd.
- Merck & Co., Inc.
- Mylan N.V.
- Novartis AG
- Panacea Biotec Ltd.
- Pfizer Inc.
- Sanofi Pasteur
- Serum Institute of India Pvt. Ltd.
- Sinovac Biotech Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 277 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
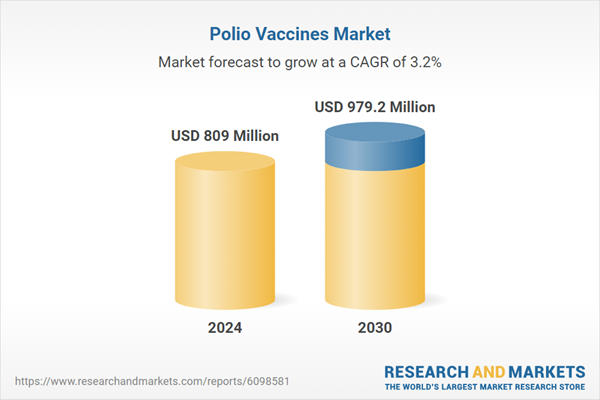
| Estimated Market Value ( USD | $ 809 Million |
| Forecasted Market Value ( USD | $ 979.2 Million |
| Compound Annual Growth Rate | 3.2% |
| Regions Covered | Global |