Global 'Preeclampsia Diagnostics' Market - Key Trends & Drivers Summarized
Why Is Early Detection of Preeclampsia Gaining Clinical Priority?
Preeclampsia, a pregnancy complication marked by high blood pressure and potential organ damage, is a leading cause of maternal and neonatal morbidity worldwide. Early detection is critical, as untreated preeclampsia can escalate into life-threatening eclampsia or lead to premature births. As healthcare systems emphasize preventive prenatal care, diagnostic solutions for preeclampsia are receiving increased attention. Recent innovations include biomarker-based blood tests such as the sFlt-1/PlGF ratio, which enables early and reliable risk stratification. Point-of-care testing, integrated with portable analyzers and digital monitoring, is also emerging to support maternal care in both urban hospitals and remote clinics. These diagnostics are reshaping prenatal care by enabling timely intervention, thereby improving pregnancy outcomes and reducing the burden on intensive care services.Who Are the Primary Stakeholders Advancing Diagnostic Capabilities?
Stakeholders in this evolving market include medical device manufacturers, obstetric care providers, academic researchers, and public health agencies. Diagnostic companies are developing assays with higher sensitivity and specificity to detect biomarkers in serum or plasma well before clinical symptoms appear. Hospitals and prenatal clinics are integrating these tests into standard care pathways to stratify high-risk pregnancies early on. Meanwhile, research institutions are exploring genomic and proteomic signatures for potential use in next-gen diagnostics. Global health agencies such as WHO and UNICEF are promoting maternal screening programs, particularly in low-income countries where preeclampsia-related mortality remains high. Collaborations between public entities and private players are expanding access to affordable diagnostics through subsidized programs and mobile health initiatives.How Is the Global Market Adapting Across Regions?
Regional adoption of preeclampsia diagnostics varies based on healthcare infrastructure, maternal mortality rates, and regulatory readiness. North America and Western Europe are leading adopters, with established screening protocols and widespread insurance coverage supporting advanced testing. In Asia-Pacific, urban areas in China, Japan, and India are seeing rapid implementation of biomarker assays, driven by rising awareness and the expansion of maternal care infrastructure. Sub-Saharan Africa and parts of Latin America face challenges with access and affordability, but mobile clinics and telehealth platforms are bridging some gaps. Global initiatives are encouraging broader deployment of point-of-care diagnostic tools in these underserved regions, supported by donations and technology transfer from developed markets. These regional variations reflect the diverse maturity levels and urgency surrounding maternal health priorities.What Is Driving the Expansion of Diagnostic Solutions?
The growth in the preeclampsia diagnostics market is driven by several factors rooted in clinical demand, healthcare policy, and technological evolution. Rising awareness of maternal health risks and early intervention benefits is pushing obstetricians and gynecologists to adopt biomarker-based tests as part of routine prenatal care. Advances in immunoassays, biosensors, and microfluidics have enabled the development of fast, accurate, and scalable diagnostic platforms. The increasing prevalence of high-risk pregnancies - due to factors such as older maternal age, obesity, and pre-existing hypertension - is amplifying the need for reliable screening. Supportive health policies and prenatal care incentives in both developed and developing countries are facilitating wider market access. Furthermore, partnerships between diagnostic developers and healthcare NGOs are enabling cost-effective deployment of testing solutions in resource-limited settings, thereby enhancing global penetration and impact.Report Scope
The report analyzes the Preeclampsia Diagnostics market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Test Type (Blood Tests, Urine Analysis, Other Test Types); Product Type (Instruments, Consumables); End-User (Hospitals, Specialty Clinics, Diagnostic Centers, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Some of the 43 companies featured in this Preeclampsia Diagnostics market report include -
- Abbott Laboratories
- ACON Laboratories, Inc.
- Agilent Technologies, Inc.
- Bayer AG
- Beckman Coulter, Inc.
- Becton, Dickinson and Company
- bioMérieux SA
- Bio-Rad Laboratories, Inc.
- Diabetomics, Inc.
- DRG Instruments GmbH
- F. Hoffmann-La Roche Ltd
- Illumina, Inc.
- Metabolomic Diagnostics Ltd.
- Natera, Inc.
- PerkinElmer Inc.
- QuidelOrtho Corporation
- Sera Prognostics, Inc.
- Siemens Healthineers AG
- Thermo Fisher Scientific Inc.
- Trinity Biotech plc
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Blood Tests segment, which is expected to reach US$2.2 Billion by 2030 with a CAGR of a 11%. The Urine Analysis segment is also set to grow at 7.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $556.1 Million in 2024, and China, forecasted to grow at an impressive 13.4% CAGR to reach $727.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Preeclampsia Diagnostics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Preeclampsia Diagnostics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Preeclampsia Diagnostics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AB SKF, Barnes Industries Inc., Bosch Rexroth AG, Del-Tron Precision, Inc., Dynatect Manufacturing, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 43 Featured):
- Abbott Laboratories
- ACON Laboratories, Inc.
- Agilent Technologies, Inc.
- Bayer AG
- Beckman Coulter, Inc.
- Becton, Dickinson and Company
- bioMérieux SA
- Bio-Rad Laboratories, Inc.
- Diabetomics, Inc.
- DRG Instruments GmbH
- F. Hoffmann-La Roche Ltd
- Illumina, Inc.
- Metabolomic Diagnostics Ltd.
- Natera, Inc.
- PerkinElmer Inc.
- QuidelOrtho Corporation
- Sera Prognostics, Inc.
- Siemens Healthineers AG
- Thermo Fisher Scientific Inc.
- Trinity Biotech plc
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- ACON Laboratories, Inc.
- Agilent Technologies, Inc.
- Bayer AG
- Beckman Coulter, Inc.
- Becton, Dickinson and Company
- bioMérieux SA
- Bio-Rad Laboratories, Inc.
- Diabetomics, Inc.
- DRG Instruments GmbH
- F. Hoffmann-La Roche Ltd
- Illumina, Inc.
- Metabolomic Diagnostics Ltd.
- Natera, Inc.
- PerkinElmer Inc.
- QuidelOrtho Corporation
- Sera Prognostics, Inc.
- Siemens Healthineers AG
- Thermo Fisher Scientific Inc.
- Trinity Biotech plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 376 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
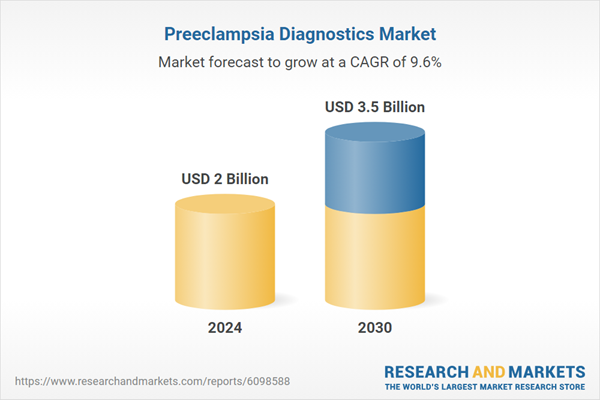
| Estimated Market Value ( USD | $ 2 Billion |
| Forecasted Market Value ( USD | $ 3.5 Billion |
| Compound Annual Growth Rate | 9.6% |
| Regions Covered | Global |