Global Soft Mist Inhalers Market - Key Trends & Drivers Summarized
Is the Soft Mist Revolution in Pulmonary Drug Delivery Just Getting Started?
Soft mist inhalers (SMIs) are redefining the paradigm of inhalation therapy by addressing the limitations of traditional dry powder inhalers (DPIs) and pressurized metered-dose inhalers (pMDIs). These handheld drug delivery devices generate a slow-moving, long-duration mist without relying on propellants or high inspiratory effort, making them especially effective for patients with compromised lung function. Unlike pMDIs that require hand-breath coordination or DPIs that demand high inspiratory flow rates, SMIs offer consistent aerosol deposition deep into the lungs, improving therapeutic outcomes in chronic obstructive pulmonary disease (COPD), asthma, and emerging pulmonary indications.SMIs are gaining widespread attention in both adult and pediatric respiratory care owing to their precise dosing capabilities and reduced oropharyngeal deposition. These advantages translate into improved drug delivery efficiency and reduced systemic side effects. The technology has been particularly impactful for elderly patients and children who often struggle with inhaler technique. By eliminating the need for a propellant, SMIs also align with environmental regulations banning high-GWP propellants like hydrofluoroalkanes (HFAs), giving manufacturers a sustainable alternative. Additionally, SMIs enable the use of a wider range of formulations, including aqueous-based and biologic drug solutions, thereby expanding the inhalable drug pipeline.
Why Are SMIs Becoming the Go-To Solution for Next-Gen Respiratory Formulations?
The increasing clinical emphasis on lung deposition efficiency, patient adherence, and precision dosing is driving pharmaceutical companies to invest in soft mist platforms. Unlike other inhalation devices where drug losses can be significant, SMIs deliver high-fine particle fractions consistently across a broad spectrum of patient profiles. This makes them ideal for low-dose, high-potency drugs such as bronchodilators, corticosteroids, and combination therapies used in long-term respiratory management. Additionally, the compatibility of SMIs with biologics and novel molecular entities is allowing companies to explore indications beyond asthma and COPD, including pulmonary hypertension, cystic fibrosis, and post-COVID lung rehabilitation.From a design standpoint, SMIs offer flexibility in both reusable and disposable formats. Reusable SMI devices with interchangeable cartridges are being adopted in clinical trials and commercial programs to reduce plastic waste and improve long-term adherence. Digital integration is another key advancement. New-generation SMIs are being embedded with dose counters, Bluetooth connectivity, and companion apps that remind patients to take their medication, track inhalation history, and share adherence data with healthcare providers. This data-driven model of respiratory care is making SMIs a cornerstone in the push toward personalized and accountable therapy.
How Are R&D Advancements and Regulatory Approvals Expanding the SMI Market Landscape?
Pharmaceutical R&D pipelines are increasingly favoring SMI-compatible formulations as regulatory bodies such as the FDA and EMA express greater interest in devices that enhance treatment adherence and reduce variability. A surge in clinical trials featuring SMI-delivered therapies is evident across North America, Europe, and Asia. Companies are leveraging computational fluid dynamics (CFD) simulations, in vitro-in vivo correlation (IVIVC) models, and human factor engineering studies to design user-centric SMIs that optimize performance while minimizing operational complexity. As more therapies transition from intravenous to inhaled formats, SMIs are being positioned as the ideal delivery system, especially for systemic or targeted lung therapies.On the manufacturing front, SMIs are benefiting from improved microfluidic nozzle designs, battery-free actuation mechanisms, and durable biocompatible materials that lower cost and extend device lifecycle. Regulatory convergence on human factor studies, dose accuracy standards, and device usability testing is reducing approval timelines for SMI-based drug-device combinations. Moreover, the increasing role of contract development and manufacturing organizations (CDMOs) with inhalation device expertise is lowering entry barriers for smaller pharma companies and accelerating time-to-market. These factors are contributing to global market expansion, with emerging markets such as India, Brazil, and Southeast Asia witnessing rapid adoption of SMI-based therapies.
What's Fueling the Acceleration of the Global Soft Mist Inhalers Market?
The growth in the soft mist inhalers market is driven by several factors, including demographic shifts, evolving clinical practices, and strategic regulatory support. The aging global population and rising prevalence of chronic respiratory diseases like COPD, asthma, and idiopathic pulmonary fibrosis are central to SMI adoption. With older adults often unable to use pMDIs or DPIs effectively, SMIs provide a reliable alternative that enhances therapeutic efficacy without compromising user experience. Increasing diagnosis rates and improved access to healthcare in emerging economies are also broadening the patient base eligible for SMI therapy.The transition toward greener healthcare is another critical driver. With global mandates to phase out harmful propellants and reduce carbon footprints, SMIs offer an environmentally viable solution. Their compatibility with aqueous drug formulations and lack of reliance on external power sources adds to their sustainable appeal. Lastly, the shift toward integrated digital therapeutics is enabling real-time monitoring, adherence tracking, and telemedicine-compatible care pathways - all of which are readily supported by connected SMI platforms. These drivers, coupled with continuous device innovation and growing pharma partnerships, are propelling soft mist inhalers into the mainstream of respiratory care innovation.
Report Scope
The report analyzes the Soft Mist Inhalers market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Disposable Soft Mist Inhalers, Reusable Soft Mist Inhalers); Application (Asthma Application, COPD Application, Other Applications); Age Group (Adults Age Group, Pediatrics Age Group); End-Use (Hospitals End-Use, Clinics End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
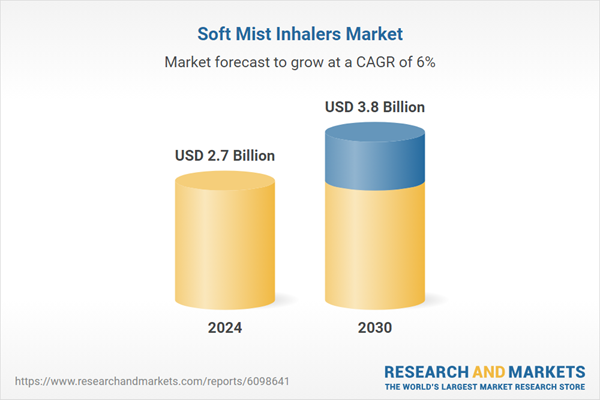
- Market Growth: Understand the significant growth trajectory of the Disposable Soft Mist Inhalers segment, which is expected to reach US$2.7 Billion by 2030 with a CAGR of a 7%. The Reusable Soft Mist Inhalers segment is also set to grow at 3.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $723.6 Million in 2024, and China, forecasted to grow at an impressive 9.7% CAGR to reach $776.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Soft Mist Inhalers Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Soft Mist Inhalers Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Soft Mist Inhalers Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Azelis, BMD Materials, BuGuCh & Partners, CDH Fine Chemical, Chemicals Global and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Soft Mist Inhalers market report include:
- 3M
- Aero Pump GmbH
- Boehringer Ingelheim
- Changzhou DSB Medical Co., Ltd.
- Chiesi Farmaceutici S.p.A.
- Harro Höfliger
- Hikma Pharmaceuticals
- Invox Pharma
- Merck & Co., Inc.
- Merxin Ltd
- Mundipharma
- Novartis AG
- Recipharm AB
- Resyca BV
- Roche Holding AG
- Suzhou Singmed Medical Device Co.
- Teva Pharmaceutical Industries
- Ursatec GmbH
- Viatris Inc.
- WERRTA GmbH
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3M
- Aero Pump GmbH
- Boehringer Ingelheim
- Changzhou DSB Medical Co., Ltd.
- Chiesi Farmaceutici S.p.A.
- Harro Höfliger
- Hikma Pharmaceuticals
- Invox Pharma
- Merck & Co., Inc.
- Merxin Ltd
- Mundipharma
- Novartis AG
- Recipharm AB
- Resyca BV
- Roche Holding AG
- Suzhou Singmed Medical Device Co.
- Teva Pharmaceutical Industries
- Ursatec GmbH
- Viatris Inc.
- WERRTA GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 459 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 2.7 Billion |
| Forecasted Market Value ( USD | $ 3.8 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |