Global Software as a Medical Device Market - Key Trends & Drivers Summarized
Can Code Alone Cure? Why Software as a Medical Device Is Rewriting Healthcare Norms
Software as a Medical Device (SaMD) is revolutionizing healthcare by allowing software programs - independent of any hardware - to perform functions such as diagnosis, treatment recommendation, or disease monitoring. Unlike traditional software embedded within devices, SaMD operates autonomously on mobile devices, cloud platforms, or desktop applications, enabling remote patient management and data-driven clinical decision-making. From detecting cardiac arrhythmias via smartphone ECG algorithms to optimizing insulin dosing through predictive diabetes software, SaMD is transforming how healthcare is delivered, particularly in chronic disease management, mental health, radiology, and digital therapeutics.What makes SaMD particularly disruptive is its ability to deliver clinical functionality at scale without the need for physical manufacturing. This agility allows rapid updates, iterative improvements, and real-time data analysis - all essential in managing complex conditions like oncology, neurology, and rare diseases. Additionally, the COVID-19 pandemic accelerated the validation of SaMD tools for remote diagnostics and digital triage, paving the way for regulatory agencies like the FDA and EMA to establish fast-track pathways for their approval. The demand for personalized, non-invasive, and continuous monitoring solutions has never been higher, positioning SaMD as a linchpin in the evolving continuum of care.
Why Are Stakeholders from Pharma to Payers Rallying Behind SaMD?
The convergence of cloud computing, artificial intelligence, and mobile technologies has created a fertile environment for SaMD deployment across both clinical and consumer settings. Pharmaceutical companies are increasingly integrating SaMD into their drug development and post-marketing surveillance strategies, using digital biomarkers and real-world data to enhance therapeutic efficacy and monitor patient adherence. These software solutions are also being bundled with companion diagnostics, connected drug delivery devices, and wearable sensors to form comprehensive digital health ecosystems. For payers and providers, SaMD offers tools for early detection, risk stratification, and intervention that can reduce hospitalization rates and improve cost efficiency.Regulatory bodies have responded by establishing frameworks that ensure patient safety without stifling innovation. The FDA's Digital Health Software Precertification Program, the EU Medical Device Regulation (MDR), and Japan's Pharmaceuticals and Medical Devices Agency (PMDA) have introduced streamlined pathways tailored for SaMD. These frameworks emphasize post-market surveillance, user-centric design, and real-world performance validation. SaMD's ability to operate independently of hardware allows it to scale rapidly across geographies, making it especially effective in resource-constrained healthcare settings. As value-based care models expand globally, SaMD is poised to become a core enabler of proactive, personalized healthcare.
How Are AI, Machine Learning, and Real-World Data Powering SaMD Evolution?
Artificial intelligence and machine learning are at the heart of SaMD innovation. These technologies allow software to analyze vast datasets, identify patterns, and make predictive assessments with high precision. In radiology, AI-powered SaMD can detect abnormalities such as tumors or fractures from imaging scans with accuracy comparable to or exceeding human specialists. In cardiology, ML algorithms assess ECG signals for arrhythmias or heart failure indicators. In behavioral health, SaMD solutions are being trained to detect early signs of anxiety or depression through speech patterns, facial expressions, and digital interaction data. These capabilities are not just enhancing diagnostic accuracy but enabling earlier and more personalized interventions.Real-world data (RWD) and real-world evidence (RWE) are further propelling the value proposition of SaMD. As software platforms integrate data from wearables, electronic health records, and patient-reported outcomes, they become powerful tools for continuous learning and real-time risk assessment. Developers are leveraging cloud infrastructure and federated learning models to ensure data privacy while improving algorithmic performance across diverse populations. This feedback loop is enabling regulatory agencies to approve adaptive algorithms - SaMD products that evolve post-approval based on new evidence. The integration of cybersecurity, HIPAA compliance, and user authentication systems ensures that these solutions remain secure and trustworthy.
What's Fueling the Exponential Rise of Software as a Medical Device?
The growth in the software as a medical device market is driven by several synergistic forces across the digital health landscape. Chief among them is the escalating demand for remote care and self-management tools, especially in chronic and lifestyle-driven diseases. With patients increasingly relying on mobile apps and connected platforms to monitor their health, SaMD is bridging the gap between clinical settings and home-based care. Moreover, the proliferation of smartphones and cloud infrastructure in emerging markets is democratizing access to SaMD, allowing underserved populations to benefit from AI-driven healthcare solutions.Pharmaceutical and medtech companies are rapidly incorporating SaMD into their go-to-market strategies, viewing it as a means to extend the therapeutic impact of their products and gather post-market insights. Meanwhile, hospitals and payers are leveraging SaMD for triage automation, patient engagement, and early detection to reduce the burden on emergency departments and improve care coordination. Finally, venture capital and private equity investment in SaMD startups is at an all-time high, signaling strong market confidence. As healthcare systems evolve toward personalized, data-centric, and preventive care models, SaMD is positioned to serve as both a clinical tool and a strategic differentiator in global healthcare delivery.
Report Scope
The report analyzes the Software as a Medical Device market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Deployment (Cloud, On-Premise); Application (Screening & Diagnosis, Monitoring & Alerting, Chronic Disease Management, Digital Therapeutics, Other Applications); End-Use (Hospitals & Clinics, Home Care Settings, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Cloud Deployment segment, which is expected to reach US$32 Million by 2030 with a CAGR of a 14.1%. The On-Premise Deployment segment is also set to grow at 18.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $6 Million in 2024, and China, forecasted to grow at an impressive 20.7% CAGR to reach $11.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Software as a Medical Device Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Software as a Medical Device Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Software as a Medical Device Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 3M, Aero Pump GmbH, Boehringer Ingelheim, Changzhou DSB Medical Co., Ltd., Chiesi Farmaceutici S.p.A. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Software as a Medical Device market report include:
- AliveCor
- Apple Inc.
- Arterys Inc.
- Biofourmis
- Brainlab
- Cerner Corporation
- Digital Diagnostics Inc.
- GE Healthcare
- Google Health
- Health Catalyst
- IBM Watson Health
- iSchemaView Inc.
- Medtronic plc
- MindMaze
- Microsoft Health
- Philips Healthcare
- Qlarity Imaging LLC
- Samsung Health
- Siemens Healthineers
- Viz.ai
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AliveCor
- Apple Inc.
- Arterys Inc.
- Biofourmis
- Brainlab
- Cerner Corporation
- Digital Diagnostics Inc.
- GE Healthcare
- Google Health
- Health Catalyst
- IBM Watson Health
- iSchemaView Inc.
- Medtronic plc
- MindMaze
- Microsoft Health
- Philips Healthcare
- Qlarity Imaging LLC
- Samsung Health
- Siemens Healthineers
- Viz.ai
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 379 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
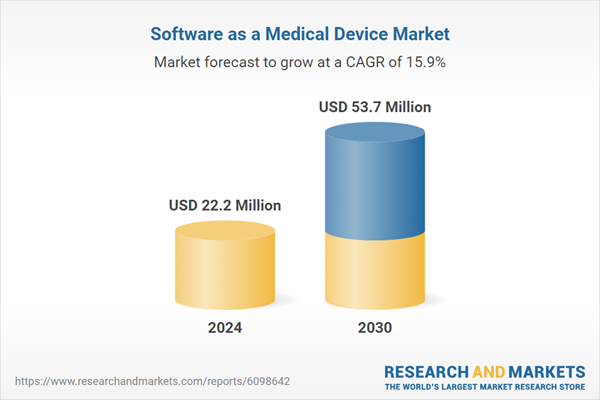
| Estimated Market Value ( USD | $ 22.2 Million |
| Forecasted Market Value ( USD | $ 53.7 Million |
| Compound Annual Growth Rate | 15.9% |
| Regions Covered | Global |