Global Endoscope Drying Cabinets Market - Key Trends & Drivers Summarized
Why Are Endoscope Drying Cabinets Becoming Essential in Infection Control Protocols?
Endoscope drying cabinets are critical to the safe storage and reprocessing of flexible endoscopes after high-level disinfection (HLD), ensuring they remain dry, contamination-free, and ready for use in clinical procedures. Inadequate drying has been linked to microbial growth, biofilm formation, and cross-contamination risks, particularly in gastrointestinal, respiratory, and urological procedures. Drying cabinets eliminate residual moisture in lumens and external surfaces by circulating filtered, temperature-controlled air, often combined with HEPA filtration and individual channel airflow technology.As hospital-acquired infections (HAIs) and multidrug-resistant organisms (MDROs) draw increasing scrutiny, regulatory bodies such as the U.S. CDC, ECRI, and European Society of Gastrointestinal Endoscopy are recommending or mandating the use of automated drying and storage systems. Technological advancements now include data logging, RFID scope tracking, touchless loading systems, and integration with automated endoscope reprocessors (AERs) for end-to-end workflow optimization. Leading manufacturers like Steelco, Wassenburg Medical, Olympus, and Getinge are expanding their portfolio to include vertical drying cabinets, scope segregation by specialty, and smart IoT-enabled models for remote monitoring and compliance verification.
Which Healthcare Settings and Regulations Are Driving Cabinet Adoption?
Hospitals, outpatient endoscopy centers, and specialty surgical clinics are the primary users of endoscope drying cabinets. The surge in diagnostic and therapeutic endoscopy procedures - particularly in gastroenterology, ENT, and bronchoscopy - is increasing the volume of scopes needing reprocessing, necessitating efficient drying and storage solutions. Accreditation agencies and infection control committees are enforcing stricter guidelines around reprocessing protocols, requiring documented proof of drying cycles, scope segregation by infection risk level, and adherence to storage duration limits (e.g., 72 or 168 hours depending on jurisdiction).Regions with stringent healthcare quality standards - such as the U.S., Germany, Japan, and South Korea - are leading adoption, while fast-developing markets in Southeast Asia and the Middle East are beginning to integrate drying cabinets as part of broader infection control upgrades. Additionally, post-pandemic infection control reforms are prompting healthcare systems to invest in touchless workflows and automated traceability systems, reinforcing demand for cabinets with integrated audit trails, user access controls, and scope-specific drying configurations.
The Growth in the Endoscope Drying Cabinets Market Is Driven by Several Factors…
The growth in the endoscope drying cabinets market is driven by rising procedural volumes, stricter infection control regulations, and technological innovations that reduce contamination risk and improve operational efficiency. Advancements in channel-specific airflow, HEPA-based drying, and real-time humidity monitoring are ensuring consistent drying results across varying scope types and models. Digital connectivity features - such as RFID-enabled inventory control, automated scope ID tracking, and cloud-based documentation - are addressing traceability mandates and aiding accreditation readiness.On the end-use side, increasing use of flexible endoscopes in minimally invasive procedures is accelerating demand for reprocessing infrastructure. Hospitals are expanding central sterile services departments (CSSDs) to include advanced drying and storage capabilities that reduce infection risks while optimizing reprocessing throughput. Additionally, healthcare facility investments in infection-resistant design and workflow automation are incorporating drying cabinets as core infrastructure in new endoscopy units. These drivers are cementing endoscope drying cabinets as a mandatory component in modern sterile reprocessing workflows.
Report Scope
The report analyzes the Endoscope Drying Cabinets market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Double Door Cabinet, Single Door Cabinet, Benchtop, Wall-mounted, Floor-standing, Other Products); Size (8 Endoscope, 12 Endoscope, 16 Endoscope, Other Sizes); End-User (Ambulatory Surgery Centers, Hospitals, Clinics, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Double Door Cabinet segment, which is expected to reach US$123 Million by 2030 with a CAGR of a 9.5%. The Single Door Cabinet segment is also set to grow at 8.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $66.1 Million in 2024, and China, forecasted to grow at an impressive 12.4% CAGR to reach $82.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Endoscope Drying Cabinets Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Endoscope Drying Cabinets Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Endoscope Drying Cabinets Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as A TEC Production & Service GmbH, AAF International, Alfa Laval AB, Babcock & Wilcox Enterprises, Inc., Camfil AB and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Endoscope Drying Cabinets market report include:
- Arc Healthcare Solutions
- AT-OS
- Belimed AG
- Cantel Medical (now part of STERIS)
- Capsa Healthcare
- Choyang Medical Industry Inc.
- Ecolab (Soluscope)
- Elmed Electronics & Medical Industry & Trade Inc.
- Escad Medical
- Gallay Medical & Scientific
- Getinge AB
- Harloff
- HOYA Corporation (Wassenburg)
- LTE Scientific Ltd
- MEDIVATORS Inc. (Cantel Medical)
- Olympus Corporation
- Smartline Medical Pty Ltd
- Steelco S.p.A.
- STERIS plc
- Torvan Medical
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Arc Healthcare Solutions
- AT-OS
- Belimed AG
- Cantel Medical (now part of STERIS)
- Capsa Healthcare
- Choyang Medical Industry Inc.
- Ecolab (Soluscope)
- Elmed Electronics & Medical Industry & Trade Inc.
- Escad Medical
- Gallay Medical & Scientific
- Getinge AB
- Harloff
- HOYA Corporation (Wassenburg)
- LTE Scientific Ltd
- MEDIVATORS Inc. (Cantel Medical)
- Olympus Corporation
- Smartline Medical Pty Ltd
- Steelco S.p.A.
- STERIS plc
- Torvan Medical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 390 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
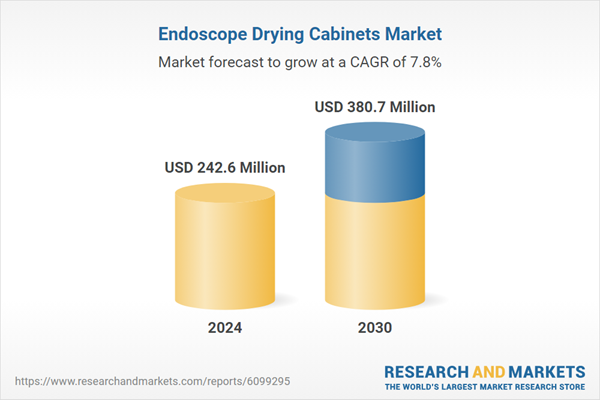
| Estimated Market Value ( USD | $ 242.6 Million |
| Forecasted Market Value ( USD | $ 380.7 Million |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Global |