Global hERG Screening Market - Key Trends & Drivers Summarized
Why Is hERG Screening Critical in Modern Drug Development Pipelines?
hERG (human Ether-à-go-go-Related Gene) screening is a crucial safety assay in drug discovery, used to evaluate a compound's potential to block the cardiac potassium ion channel encoded by the KCNH2 gene. Inhibition of this channel can lead to QT interval prolongation and increase the risk of life-threatening cardiac arrhythmias such as Torsades de Pointes. As such, hERG screening has become a mandated component of preclinical safety pharmacology by global regulatory agencies including the FDA, EMA, and ICH.Due to its implications for cardiac safety, hERG screening is essential not only for cardiovascular drugs but also for therapeutics across oncology, CNS, and infectious diseases. Drug candidates that show potential cardiotoxicity are typically modified, deprioritized, or withdrawn early in the pipeline, saving companies millions in downstream costs. This screening is also vital in lead optimization, enabling medicinal chemists to de-risk and refine compounds before entering animal or human studies.
How Are Technologies Advancing the Accuracy and Throughput of hERG Assays?
Recent advances in high-throughput screening (HTS), patch-clamp electrophysiology, and predictive computational modeling have significantly enhanced the precision and scalability of hERG testing. Automated planar patch-clamp platforms such as QPatch and SyncroPatch allow for high-content screening of ion channel activity with reduced labor and time investment. Similarly, fluorescence-based assays and voltage-sensitive dyes provide cost-effective alternatives for early-stage compound profiling.In silico modeling and machine learning are now being applied to predict hERG liability based on chemical structure, reducing the need for exhaustive in vitro testing. The use of human iPSC-derived cardiomyocytes as a physiologically relevant platform for hERG evaluation is also gaining traction, especially under the Comprehensive In Vitro Proarrhythmia Assay (CiPA) initiative. These technologies are improving predictability and regulatory alignment in preclinical cardiotoxicity assessment.
Which Sectors and Regions Are Leading hERG Screening Demand?
Pharmaceutical and biotechnology companies represent the primary end-users of hERG screening, integrating these assays during early preclinical development and regulatory submission phases. CROs (Contract Research Organizations) are also major providers of hERG screening services, offering scalable, outsourced assay capabilities to drug developers. Academic institutions and toxicology research labs utilize hERG models for cardiac safety evaluation and channelopathy research.Geographically, North America dominates the hERG screening market, driven by intensive drug development activity, stringent regulatory requirements, and advanced laboratory infrastructure. Europe follows, with a strong focus on regulatory science and public-private drug development collaborations. Asia-Pacific is emerging as a high-growth region, especially China and India, where pharmaceutical R&D spending is rising and global drugmakers are outsourcing early toxicology studies.
The Growth in the hERG Screening Market Is Driven by Several Factors…
The growth in the hERG screening market is driven by several factors related to safety pharmacology mandates, drug development complexity, and technology-driven screening innovation. Advances in automated patch-clamp systems, human-relevant cell models, and predictive AI-based screening tools have significantly enhanced throughput, accuracy, and regulatory compliance. These innovations are reducing time-to-decision for drug safety profiling while minimizing clinical failure risks.On the end-use side, the expanding global pharmaceutical pipeline, especially in oncology and CNS drugs, is increasing the volume of compounds requiring hERG liability testing. The rise of personalized medicine and targeted therapies further necessitates precise cardiotoxicity screening. Regulatory frameworks like ICH S7B and the CiPA initiative continue to emphasize hERG as a primary biomarker of cardiac safety, ensuring that it remains integral to preclinical workflows. As safety standards and development timelines grow increasingly stringent, hERG screening is becoming indispensable in de-risking and accelerating new drug development.
Report Scope
The report analyzes the hERG Screening market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Gene KCNH2 Type, Mutant KCNH2 Type); Ion Channel (Voltage-Gated Ion Channel, Ligand-Gated Ion Channel); Application (Antiarrhythmic Application, Antipsychotic Application, Antibiotics Application, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Some of the 33 companies featured in this hERG Screening market report include -
- ApconiX Ltd.
- Aurigene Pharmaceutical Services
- Charles River Laboratories
- Creative Bioarray
- Cyprotex (Evotec)
- Eurofins Discovery
- Evotec SE
- Mediford
- Metrion Biosciences
- Molecular Devices
- Reaction Biology Corporation
- Thermo Fisher Scientific
- WuXi AppTec
- Eurofins Discovery
- Charles River Laboratories
- ApconiX Ltd.
- Creative Bioarray
- Cyprotex (Evotec)
- Mediford
- Metrion Biosciences
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Gene KCNH2 segment, which is expected to reach US$3.7 Billion by 2030 with a CAGR of a 14%. The Mutant KCNH2 segment is also set to grow at 9.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $654.8 Million in 2024, and China, forecasted to grow at an impressive 17.3% CAGR to reach $1.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global hERG Screening Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global hERG Screening Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global hERG Screening Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 33 Featured):
- ApconiX Ltd.
- Aurigene Pharmaceutical Services
- Charles River Laboratories
- Creative Bioarray
- Cyprotex (Evotec)
- Eurofins Discovery
- Evotec SE
- Mediford
- Metrion Biosciences
- Molecular Devices
- Reaction Biology Corporation
- Thermo Fisher Scientific
- WuXi AppTec
- Eurofins Discovery
- Charles River Laboratories
- ApconiX Ltd.
- Creative Bioarray
- Cyprotex (Evotec)
- Mediford
- Metrion Biosciences
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ApconiX Ltd.
- Aurigene Pharmaceutical Services
- Charles River Laboratories
- Creative Bioarray
- Cyprotex (Evotec)
- Eurofins Discovery
- Evotec SE
- Mediford
- Metrion Biosciences
- Molecular Devices
- Reaction Biology Corporation
- Thermo Fisher Scientific
- WuXi AppTec
- Eurofins Discovery
- Charles River Laboratories
- ApconiX Ltd.
- Creative Bioarray
- Cyprotex (Evotec)
- Mediford
- Metrion Biosciences
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 363 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
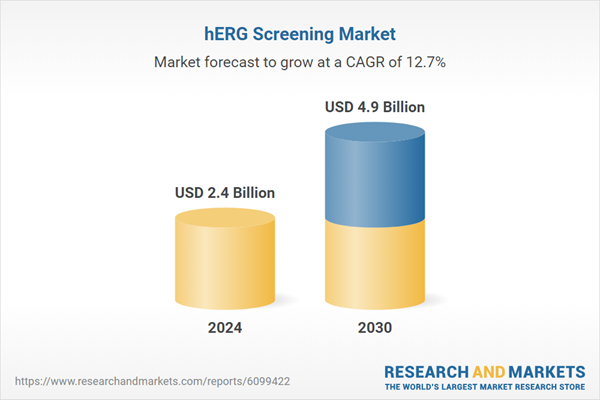
| Estimated Market Value ( USD | $ 2.4 Billion |
| Forecasted Market Value ( USD | $ 4.9 Billion |
| Compound Annual Growth Rate | 12.7% |
| Regions Covered | Global |