Global Protein Cookies Market - Key Trends & Drivers Summarized
Why Are Protein Cookies Becoming a Staple in the Functional and On-the-Go Nutrition Landscape?
Protein cookies are gaining momentum as a convenient, nutrient-dense snack alternative that bridges the gap between indulgence and functionality. Unlike conventional cookies, these products are fortified with high-quality protein sources such as whey isolate, casein, egg white protein, soy, or plant-based proteins like pea and brown rice. They cater to the growing consumer demand for clean, satiating, and fitness-aligned snacks that can support muscle maintenance, weight management, or general wellness.Consumers across demographics-from athletes and fitness enthusiasts to busy professionals and health-conscious parents-are embracing protein cookies as a portable, better-for-you snack that offers satiety and macronutrient balance. The appeal is further amplified by product claims such as gluten-free, sugar-free, keto-friendly, or vegan-certified, which align with popular dietary movements. With the global shift toward convenient functional foods, protein cookies have emerged as a fast-growing segment within the broader protein-enriched snacking category.
How Are Ingredients, Textures, and Formats Evolving to Meet Consumer Expectations?
Formulation innovation is central to the success of protein cookies, especially given consumer expectations for both nutrition and indulgence. Brands are investing in protein blends that optimize amino acid profiles while delivering soft, chewy, or crisp textures that rival traditional bakery-style cookies. To address texture challenges posed by high protein content, manufacturers are experimenting with soluble fibers, polyols, and emulsifiers to enhance moisture retention and shelf stability.Sweeteners such as erythritol, stevia, monk fruit, and allulose are being used in tandem to replace refined sugars while maintaining palatable taste profiles. Ingredient inclusions-such as chocolate chunks, nut butter swirls, and superfood infusions (e.g., flaxseed, chia, collagen)-are expanding the sensory and functional appeal. Additionally, protein cookie formats are evolving from single-serve packs and bite-sized minis to multi-packs and meal replacement SKUs, enabling broader consumption occasions including post-workout snacking, breakfast substitutes, and midday energy boosters.
Where Is Market Demand Expanding Across Demographics and Retail Channels?
Protein cookies are rapidly moving from niche fitness stores to mainstream retail aisles, driven by their growing acceptance as part of everyday health-oriented snacking. Supermarkets, convenience stores, and health food chains now stock protein cookies alongside traditional energy bars and protein shakes. E-commerce platforms are proving particularly effective for DTC brands to target fitness-conscious consumers with tailored SKUs and subscription models. Influencer-driven marketing and social media endorsements are also expanding reach among Millennials and Gen Z consumers.In North America and Europe, demand is driven by a well-established fitness culture and widespread awareness of functional nutrition. Asia-Pacific is an emerging high-growth market, particularly in urban centers where active lifestyles, diet trends, and western-style convenience foods are gaining traction. Latin America and the Middle East are beginning to see entry-level adoption, often led by multinational protein brands introducing culturally adapted formulations. Across regions, consumer education and cross-category placement-such as in bakery, health, and sports nutrition aisles-are key to driving discovery and repeat purchase.
What's Fueling the Global Growth of the Protein Cookies Market?
The growth in the global protein cookies market is driven by the rising demand for high-protein, low-sugar snacks that align with active lifestyles and evolving dietary preferences. As consumer focus shifts toward macro-conscious eating, satiety, and functional benefits, protein cookies offer a unique proposition that merges indulgence with nutritional efficacy. The convergence of fitness, convenience, and clean-label expectations has created fertile ground for protein cookies to thrive as a multipurpose snacking format.Regulatory support for protein claims, continued investment in R&D, and the diversification of plant-based protein technologies are further expanding product innovation. Retailer interest in health-forward snack offerings and rising consumer willingness to pay a premium for fortified snacks are also reinforcing market growth. With the global wellness economy showing sustained expansion and protein-centric eating gaining universal traction, protein cookies are expected to remain a pivotal product category-bridging the worlds of functional nutrition, indulgent snacking, and performance-oriented lifestyles.
Report Scope
The report analyzes the Protein Cookies market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Flavor (Fruit & Dried Fruits, Chocolate, Nuts & Seeds, Other Flavors); Protein Source (Plant Source, Animal Source); Distribution Channel (Supermarkets / Hypermarkets, Convenience Stores, Online Stores, Other Distribution Channels).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Fruit & Dried Fruits Flavor segment, which is expected to reach US$20.7 Billion by 2030 with a CAGR of a 5.6%. The Chocolate Flavor segment is also set to grow at 5.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $8.9 Billion in 2024, and China, forecasted to grow at an impressive 9.3% CAGR to reach $9.4 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Protein Cookies Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Protein Cookies Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Protein Cookies Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Adobe Inc., Amazon.com, Inc., AppLovin Corporation, Criteo S.A., Google LLC (Alphabet Inc.) and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Protein Cookies market report include:
- ADM
- Ajinomoto Co., Inc.
- Alltech
- BASF SE
- Bentoli
- Buff Bake
- Cargill Incorporated
- Chr. Hansen Holding A/S
- DSM
- DuPont
- Evonik Industries AG
- Justine's Limited
- Kemin Industries Inc.
- Lenny & Larry's LLC
- Munk Pack
- No Cow LLC
- Novozymes
- NuGo Nutrition
- Quest Nutrition
- The Hershey Company
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ADM
- Ajinomoto Co., Inc.
- Alltech
- BASF SE
- Bentoli
- Buff Bake
- Cargill Incorporated
- Chr. Hansen Holding A/S
- DSM
- DuPont
- Evonik Industries AG
- Justine's Limited
- Kemin Industries Inc.
- Lenny & Larry's LLC
- Munk Pack
- No Cow LLC
- Novozymes
- NuGo Nutrition
- Quest Nutrition
- The Hershey Company
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 377 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
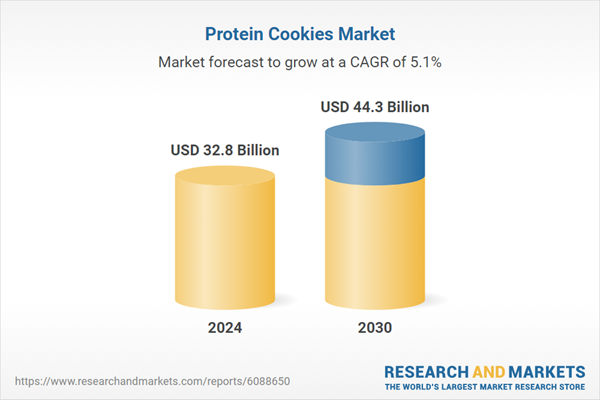
| Estimated Market Value ( USD | $ 32.8 Billion |
| Forecasted Market Value ( USD | $ 44.3 Billion |
| Compound Annual Growth Rate | 5.1% |
| Regions Covered | Global |