Global Positive Temperature Coefficient (PTC) Thermistors Market - Key Trends & Drivers Summarized
Why Are PTC Thermistors Gaining Widespread Adoption in Electronic Circuit Protection?
Positive Temperature Coefficient (PTC) thermistors are resistive components whose resistance increases significantly with a rise in temperature, making them ideal for self-regulating heating and overcurrent protection applications. Their ability to automatically limit current when overheating occurs has made them indispensable in various electronic and electrical systems, ranging from consumer devices to industrial automation. Unlike traditional fuses or relays, PTC thermistors reset automatically, ensuring uninterrupted protection and reducing the need for maintenance or component replacement.The market is witnessing robust growth due to the increasing complexity and miniaturization of electronic circuits, which demand compact and efficient protection mechanisms. In battery-powered devices, including smartphones, laptops, and power tools, PTC thermistors provide critical safeguards against thermal runaway and short circuits. Their application extends into telecom, automotive electronics, and white goods, where components are frequently exposed to voltage surges, overcurrents, or fluctuating environmental conditions. As device integration deepens across industry verticals, PTC thermistors are becoming core to the safety and reliability of embedded electronic systems.
How Are Material Advances and Design Variants Improving Thermistor Functionality?
The performance of PTC thermistors has been significantly enhanced through advancements in ceramic formulations, polymer composites, and multilayer construction techniques. Ceramic PTC thermistors, typically based on doped barium titanate, offer higher switching temperatures and are widely used in motor protection, degaussing circuits, and heating elements. Polymer PTCs, on the other hand, provide rapid response time and flexibility in form factors, making them suitable for battery packs, consumer electronics, and printed circuit boards.Design innovations are also enabling more precise control over switching characteristics, resistance tolerances, and thermal profiles. Surface mount device (SMD) formats are increasingly replacing legacy axial and radial designs due to their compactness and compatibility with automated assembly. Dual-function thermistors that offer both temperature sensing and circuit protection are also entering the market, especially for space-constrained applications in wearable electronics and compact medical devices. These advancements are making PTC thermistors not only more reliable but also more versatile across broader application areas.
Where Is Demand Surging Across Industrial, Consumer, and Automotive Applications?
In industrial automation and power electronics, PTC thermistors are used in transformers, solenoids, and relays to prevent overheating and ensure stable operation under load. HVAC systems utilize them in motor start circuits and compressor protection modules, where thermal stability and recovery are essential. In renewable energy systems-such as wind turbines and solar inverters-PTC thermistors serve as overvoltage protectors for power control units and battery storage modules.The automotive industry is one of the fastest-growing adopters of PTC thermistor technology, particularly in electric vehicles (EVs). These components regulate cabin heating systems, manage battery thermal protection, and provide current-limiting functions in charging circuits. In infotainment systems and electronic control units (ECUs), thermistors protect sensitive circuits from voltage fluctuations and overheating. In consumer electronics, demand remains strong for PTCs used in rechargeable battery packs, charging docks, and adapter circuits. The increasing proliferation of smart home devices, wearables, and IoT-enabled gadgets is expanding the base of thermistor-integrated products.
What's Driving the Global Expansion of the PTC Thermistors Market?
The growth in the global PTC thermistors market is driven by several factors, including the rising need for self-resetting circuit protection, miniaturization of electronic components, expanding EV adoption, and the integration of electronics in smart infrastructure. As devices become more compact and powerful, the thermal and electrical stresses on circuits are increasing, creating demand for intelligent protection mechanisms that are fast-acting, space-efficient, and maintenance-free. PTC thermistors are ideally positioned to meet this need due to their inherent safety, reusability, and cost-effectiveness.Increased investment in electric mobility, green energy technologies, and digital consumer electronics is fueling product innovation and application diversity. Regulatory mandates for electrical safety, combined with increasing awareness about product lifecycle reliability, are also encouraging OEMs to integrate thermistors into standard circuit architectures. With advancements in thermistor material science, packaging technology, and digital compatibility, PTC thermistors are expected to become ubiquitous across virtually every sector that relies on power regulation, thermal control, or electronic system safety.
Report Scope
The report analyzes the Positive Temperature Coefficient (PTC) Thermistors market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Ceramic PTC, Polymer PTC); Application (Consumer Electronics, Automotive, Industrial, Telecom).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Ceramic PTC segment, which is expected to reach US$521.1 Million by 2030 with a CAGR of a 4.6%. The Polymer PTC segment is also set to grow at 7.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $171.3 Million in 2024, and China, forecasted to grow at an impressive 8.9% CAGR to reach $176.5 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Positive Temperature Coefficient (PTC) Thermistors Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Positive Temperature Coefficient (PTC) Thermistors Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Positive Temperature Coefficient (PTC) Thermistors Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Arcan, Autofit GmbH, Black Bull, Curt Manufacturing LLC, Dutton-Lainson Company and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Positive Temperature Coefficient (PTC) Thermistors market report include:
- Amphenol Corporation
- Bourns, Inc.
- CYG Wayon Circuit Protection
- Fuzetec Technology Co., Ltd.
- Hansor Electronics Co., Ltd.
- HGTECH Co., Ltd.
- HIEL Electronics Co., Ltd.
- Hollyland Electronics Co., Ltd.
- Jinke Electronics Co., Ltd.
- Littelfuse, Inc.
- Murata Manufacturing Co., Ltd.
- Panasonic Corporation
- Polytronics Technology Corp.
- Sea & Land Electronics Co., Ltd.
- TDK Corporation (EPCOS)
- TE Connectivity Ltd.
- Texas Instruments Incorporated
- Thinking Electronic Industrial Co., Ltd.
- Uppermost Electronics Co., Ltd.
- Vishay Intertechnology, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Amphenol Corporation
- Bourns, Inc.
- CYG Wayon Circuit Protection
- Fuzetec Technology Co., Ltd.
- Hansor Electronics Co., Ltd.
- HGTECH Co., Ltd.
- HIEL Electronics Co., Ltd.
- Hollyland Electronics Co., Ltd.
- Jinke Electronics Co., Ltd.
- Littelfuse, Inc.
- Murata Manufacturing Co., Ltd.
- Panasonic Corporation
- Polytronics Technology Corp.
- Sea & Land Electronics Co., Ltd.
- TDK Corporation (EPCOS)
- TE Connectivity Ltd.
- Texas Instruments Incorporated
- Thinking Electronic Industrial Co., Ltd.
- Uppermost Electronics Co., Ltd.
- Vishay Intertechnology, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 281 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
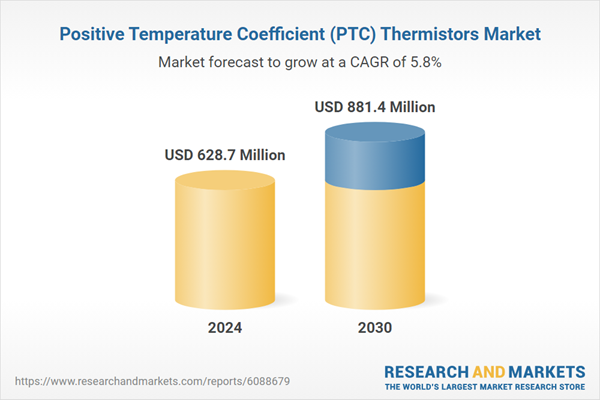
| Estimated Market Value ( USD | $ 628.7 Million |
| Forecasted Market Value ( USD | $ 881.4 Million |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |