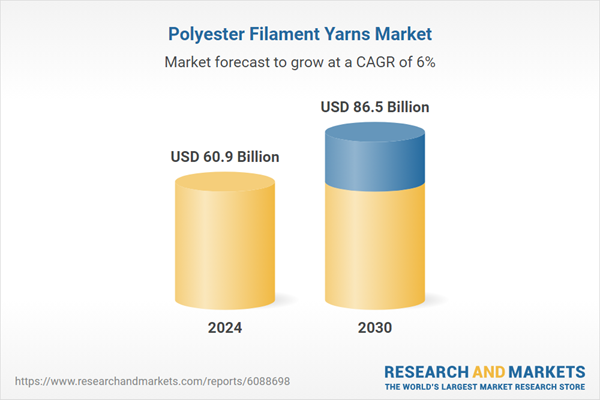
Global Polyester Filament Yarns Market - Key Trends & Drivers Summarized
Why Are Polyester Filament Yarns Central to the Global Textile and Technical Fabric Industries?
Polyester filament yarns (PFY), derived from polyethylene terephthalate (PET), are integral to both apparel and industrial textile sectors due to their exceptional strength, chemical resistance, elasticity, and cost-effectiveness. Unlike spun yarns made from staple fibers, filament yarns are continuous, enabling smoother textures and superior tensile properties. PFY variants-such as fully drawn yarn (FDY), partially oriented yarn (POY), and drawn textured yarn (DTY)-are engineered for specific applications ranging from high-performance sportswear and lingerie to seat belts, tire cords, and geotextiles.The dominance of PFY in global yarn production is underscored by its adaptability to modern weaving, knitting, and dyeing technologies, making it a backbone for mass-scale and technical textile manufacturing alike. Its resistance to shrinking, wrinkling, and mildew, coupled with its ability to accept a variety of functional finishes, has made it indispensable in both fashion and performance fabric categories. As demand for moisture-wicking, stretchable, and UV-resistant garments grows, polyester filament yarns continue to evolve to meet the needs of diverse consumer and industrial sectors.
How Are Technological Advancements Elevating Yarn Quality, Efficiency, and Sustainability?
Modern PFY production is marked by innovations in melt spinning, texturizing, and post-processing techniques that enhance yarn uniformity, reduce defects, and improve dye uptake. Advances in spinning lines-featuring high-speed draw winders, automatic doffing, and online quality sensors-have increased production efficiency and consistency across grades. Multifilament and microfilament configurations now offer tailored touch, strength, and drape properties, catering to niche segments such as silk-like textiles, functional innerwear, and technical filters.Sustainability is also shaping the PFY innovation landscape. Recycled PFY (r-PFY), made from post-consumer PET bottles or polyester waste, is gaining prominence as brands and manufacturers aim to reduce environmental impact. Closed-loop systems, zero-waste dyeing methods, and energy-optimized spinning lines are being adopted to align with ESG mandates. Additionally, bio-based PET resins are entering commercial production, offering a renewable alternative to fossil-derived polyester. With digital quality monitoring and integrated automation, PFY manufacturing is becoming smarter and more sustainable-supporting both economic scale and environmental accountability.
Where Is Demand Expanding Across Fashion, Home Textiles, and Industrial Fabric Segments?
In the apparel sector, PFY is used extensively in high-volume fashion garments, activewear, intimate apparel, and outerwear, thanks to its durability and aesthetic versatility. DTY and FDY are preferred for fabrics requiring elasticity, luster, and drape, while air-textured yarns are favored for comfort-focused applications. In home textiles, PFY finds use in curtains, upholstery, carpets, and bed linens, where fade resistance and structural integrity are key performance attributes.The industrial and technical textiles segment represents one of the fastest-growing demand areas, with PFY deployed in airbags, seatbelts, ropes, conveyor belts, tarpaulins, and reinforcement fabrics. In geotextiles and filtration media, high-tenacity PFY is valued for its load-bearing capacity and chemical resistance. Regionally, Asia-Pacific-especially China and India-dominates PFY production and consumption due to strong textile infrastructure and government support. Europe and North America, while smaller in volume, are driving demand for value-added and sustainable yarns in functional apparel and technical composites.
What's Powering the Global Growth of the Polyester Filament Yarns Market?
The growth in the global polyester filament yarns market is driven by several factors, including rising consumption of synthetic fibers in fashion and technical textiles, advancements in production technology, and the increasing emphasis on sustainability and circularity in the textile value chain. As polyester outpaces natural fibers in global fiber consumption, PFY remains a key growth engine due to its mechanical performance, cost stability, and adaptability to diverse end-uses.Increasing investments in recycling infrastructure, eco-certifications, and sustainable sourcing initiatives are creating new demand channels for recycled and low-impact PFY variants. Trade liberalization, expanding e-commerce-driven apparel markets, and the growth of domestic manufacturing hubs in Asia and Africa are further accelerating PFY adoption. Technological convergence-such as integrating antimicrobial treatments, reflective elements, and smart textile interfaces-is unlocking new functional categories. With global textiles shifting toward innovation, resilience, and environmental responsibility, polyester filament yarns are set to maintain a dominant and dynamic position in fiber markets worldwide.
Report Scope
The report analyzes the Polyester Filament Yarns market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Raw Material (Polyethylene Terephthalate, Poly-1 & 4-Cyclohexylene Dimethylene Terephthalate, Other Raw Materials); Product Type (Partially Oriented Yarn, Fully Drawn Yarn, Drawn Textured Yarn, Other Product Types); Application (Tire Cord, Mechanical Rubber Good, Non-Woven Fabric, Apparel, Industrial, Household Textile, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Polyethylene Terephthalate Raw Material segment, which is expected to reach US$55 Billion by 2030 with a CAGR of a 6.7%. The Poly-1 & 4-Cyclohexylene Dimethylene Terephthalate Raw Material segment is also set to grow at 5.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $16.6 Billion in 2024, and China, forecasted to grow at an impressive 9.6% CAGR to reach $17.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Polyester Filament Yarns Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Polyester Filament Yarns Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Polyester Filament Yarns Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as A. B. Enterprises, Arihant Chemicals, Ataman Kimya, Boz Kimya Sanayi Ticaret Ltd. Sti., Cnywat Group and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Polyester Filament Yarns market report include:
- Alok Industries Limited
- Antex S.A.
- Beximco Synthetics Limited
- Filatex India Limited
- Hangzhou Dingkai Chemical Fiber Co., Ltd.
- Hengli Group Co., Ltd.
- Hyosung Corporation
- Indorama Ventures Public Company Limited
- Jiangsu Sanfangxiang Group Co., Ltd.
- Nan Ya Plastics Corporation
- Nirmal Fibres (P) Ltd.
- Reliance Industries Limited
- Sarla Performance Fibers Limited
- Shenghong Group
- Sinopec Yizheng Chemical Fibre Company Limited
- Stein Fibers, Ltd.
- Tepar Textiles
- Thai Polyester Company Limited
- Tongkun Group Co., Ltd.
- Toray Industries, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alok Industries Limited
- Antex S.A.
- Beximco Synthetics Limited
- Filatex India Limited
- Hangzhou Dingkai Chemical Fiber Co., Ltd.
- Hengli Group Co., Ltd.
- Hyosung Corporation
- Indorama Ventures Public Company Limited
- Jiangsu Sanfangxiang Group Co., Ltd.
- Nan Ya Plastics Corporation
- Nirmal Fibres (P) Ltd.
- Reliance Industries Limited
- Sarla Performance Fibers Limited
- Shenghong Group
- Sinopec Yizheng Chemical Fibre Company Limited
- Stein Fibers, Ltd.
- Tepar Textiles
- Thai Polyester Company Limited
- Tongkun Group Co., Ltd.
- Toray Industries, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 390 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 60.9 Billion |
| Forecasted Market Value ( USD | $ 86.5 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |