Global Nucleic Acid Quantitation Kits Market - Key Trends & Drivers Summarized
Is Precision Quantitation the Missing Link in High-Throughput Genomics?
Nucleic acid quantitation kits play a pivotal role in molecular biology workflows by enabling accurate measurement of DNA and RNA concentrations in research, diagnostics, therapeutics, and forensic applications. These kits - typically based on absorbance (UV spectrophotometry), fluorescence, or colorimetric assays - are essential for quality control in sample preparation prior to PCR, qPCR, next-generation sequencing (NGS), microarray analysis, and gene expression profiling. As demand for high-integrity nucleic acid inputs increases across genomics-driven fields, quantitation kits have become indispensable for ensuring reproducibility, sensitivity, and downstream assay fidelity.In clinical genomics and diagnostic laboratories, nucleic acid quantification is a frontline process for evaluating sample purity and suitability. High-throughput laboratories handling biopsy-derived RNA, cfDNA, or viral RNA require fast, reliable, and scalable quantification tools with high sensitivity and low sample consumption. Kits employing fluorescence-based dyes such as PicoGreen®, RiboGreen®, or Qubit® fluorophores are favored for their ability to selectively bind nucleic acids with minimal interference from contaminants like phenol, proteins, or salts. In parallel, miniaturized platforms integrated with microfluidic devices are enabling real-time quantitation within automated sample-to-answer systems.
Why Is the Market Shifting Toward Ultra-Sensitive and Workflow-Compatible Quantitation Tools?
The growing complexity of genomics and transcriptomics experiments is amplifying the need for precise nucleic acid measurement at increasingly low concentrations. Applications such as single-cell sequencing, liquid biopsy analysis, and low-input RNA-seq require quantitation methods capable of detecting nanogram or picogram-level DNA/RNA with high specificity. This is steering the market toward fluorescence- and dye-based kits that offer selective binding, broad dynamic range, and compatibility with multiplex workflows. These kits support critical decision-making in library preparation, normalization, and fragment analysis for NGS and CRISPR editing platforms.Workflow integration is another key demand driver. Quantitation kits are increasingly designed for compatibility with robotic liquid handlers, multi-well plate formats, and LIMS software, enabling seamless incorporation into automated laboratory pipelines. Time-to-result, reagent stability, and minimal hands-on time are becoming crucial features in core facilities and diagnostic centers. Manufacturers are also innovating with lyophilized reagents, multiplexing capacity, and combined quantification-purity assays to meet the requirements of both high-throughput environments and field-based research. Cloud-based data logging and instrument connectivity are further enhancing quality assurance and traceability in regulated genomics workflows.
How Are New Application Frontiers and Regulatory Frameworks Driving Innovation?
The expansion of molecular diagnostics, personalized medicine, and synthetic biology is redefining the applications of nucleic acid quantitation. In diagnostics, the growing use of nucleic acid amplification tests (NAATs) for infectious diseases and oncology is prompting the adoption of rapid and clinically validated quantitation kits. In cell and gene therapy, where dosing and vector quality depend on accurate nucleic acid input, quantitation kits serve as critical control tools. Regulatory agencies such as the FDA and EMA are also emphasizing analytical validation of quantitation steps in diagnostic test kits and therapeutic workflows.In agricultural biotechnology, food traceability, and biosurveillance, portable quantitation kits with simplified protocols are enabling nucleic acid analysis in non-laboratory settings. Kits compatible with isothermal amplification or CRISPR-based diagnostics are being developed to support field-based and point-of-care applications. Meanwhile, academic and industrial researchers working in epigenetics, metagenomics, and non-coding RNA studies require kits that are optimized for fragmented, degraded, or chemically modified nucleic acids. These requirements are accelerating innovation in reagents, buffer systems, and signal amplification techniques to enhance accuracy and adaptability.
What's Driving the Accelerated Growth of the Nucleic Acid Quantitation Kits Market?
The growth in the nucleic acid quantitation kits market is driven by a combination of surging demand for molecular diagnostics, expanding applications in high-throughput sequencing, and rising investments in biopharma R&D. The explosion of RNA- and DNA-based therapeutics - including mRNA vaccines, antisense oligonucleotides, and gene editing therapies - has created new imperatives for precise nucleic acid quality control. Biopharmaceutical companies and contract research organizations (CROs) are investing in standardized quantitation kits to meet regulatory expectations and ensure batch-to-batch consistency in therapeutic development.In parallel, the democratization of genomics is fueling kit adoption in academic labs, diagnostic startups, and personalized health companies. As the cost of sequencing falls and new applications emerge, nucleic acid quantitation is becoming a routine and recurring need across diverse sectors. Global health programs focused on pathogen surveillance, antimicrobial resistance, and pandemic preparedness are also driving procurement of field-deployable quantitation kits for decentralized molecular testing.
With innovations in detection chemistry, miniaturization, and software integration, nucleic acid quantitation kits are evolving from standalone reagents into workflow enablers. As the life sciences ecosystem continues to scale in volume and complexity, these kits are expected to see sustained demand growth and technological advancement across clinical, research, agricultural, and biomanufacturing domains.
Report Scope
The report analyzes the Nucleic Acid Quantitation Kits market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (DNA Quantitation Kits, RNA Quantitation Kits); Application (Hospital Laboratories, Reference Laboratories, Academic Research Laboratories, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the DNA Quantitation Kits segment, which is expected to reach US$1.5 Billion by 2030 with a CAGR of a 6.4%. The RNA Quantitation Kits segment is also set to grow at 3.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $456.5 Million in 2024, and China, forecasted to grow at an impressive 8.8% CAGR to reach $467.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Nucleic Acid Quantitation Kits Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Nucleic Acid Quantitation Kits Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Nucleic Acid Quantitation Kits Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Commonwealth Fusion Systems, Electric Fusion Systems, First Light Fusion, Fuse Energy, Fusion Energy Insights and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Nucleic Acid Quantitation Kits market report include:
- AAT Bioquest, Inc.
- Abcam plc
- Agilent Technologies
- Analytik Jena AG
- Bio-Rad Laboratories
- Biotium, Inc.
- Enzo Life Sciences
- GE Healthcare
- Invitrogen (Thermo Fisher Scientific)
- Merck KGaA
- New England Biolabs
- PerkinElmer, Inc.
- Promega Corporation
- Qiagen N.V.
- Roche Diagnostics
- Sigma-Aldrich (Merck)
- Thermo Fisher Scientific
- TriLink BioTechnologies
- Vector Laboratories
- VWR International
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AAT Bioquest, Inc.
- Abcam plc
- Agilent Technologies
- Analytik Jena AG
- Bio-Rad Laboratories
- Biotium, Inc.
- Enzo Life Sciences
- GE Healthcare
- Invitrogen (Thermo Fisher Scientific)
- Merck KGaA
- New England Biolabs
- PerkinElmer, Inc.
- Promega Corporation
- Qiagen N.V.
- Roche Diagnostics
- Sigma-Aldrich (Merck)
- Thermo Fisher Scientific
- TriLink BioTechnologies
- Vector Laboratories
- VWR International
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 284 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
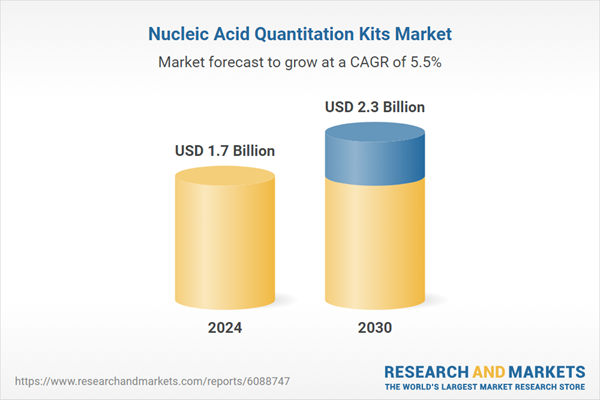
| Estimated Market Value ( USD | $ 1.7 Billion |
| Forecasted Market Value ( USD | $ 2.3 Billion |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |