Global DNA Vaccines Market - Key Trends & Drivers Summarized
Why Are DNA Vaccines Emerging as a Game-Changer in Preventive Medicine?
DNA vaccines are rapidly gaining prominence in the global vaccine landscape due to their unique advantages over traditional vaccines, offering a powerful blend of safety, stability, and adaptability. Unlike conventional vaccines that use inactivated pathogens or protein subunits, DNA vaccines introduce genetically engineered plasmids containing the DNA sequence encoding the antigen of interest. Once administered, the host cells produce the antigen internally, thereby stimulating both humoral and cellular immune responses. This mechanism mimics natural infection more closely and enhances long-term immunity. One of the primary attractions of DNA vaccines is their non-infectious nature, eliminating the risk of reversion to virulence - a concern with live-attenuated vaccines. They are also less prone to degradation, allowing for room temperature storage and easing distribution, particularly in resource-limited settings. Moreover, DNA vaccines can be rapidly developed and manufactured at scale, a feature that proved especially valuable during the COVID-19 pandemic and holds promise for responding to future outbreaks. Their modular design makes them highly adaptable for targeting emerging variants and different pathogens altogether. As global health systems strive to improve pandemic preparedness and vaccination equity, DNA vaccines are increasingly seen as a flexible, next-generation solution capable of transforming disease prevention across both human and veterinary medicine.How Are Advances in Delivery Technology and Genetic Engineering Fueling DNA Vaccine Development?
Technological innovation is playing a pivotal role in enhancing the efficacy and delivery of DNA vaccines, which historically faced challenges in terms of weak immunogenicity in humans. One of the most significant breakthroughs has been the development of advanced delivery systems such as electroporation, where a small electrical pulse creates temporary pores in cell membranes to facilitate DNA uptake. Needle-free injectors and nanoparticle carriers are also being explored to improve cellular delivery and patient compliance. Furthermore, enhancements in plasmid design - such as codon optimization, strong promoters, and inclusion of immune-stimulatory elements - have significantly boosted gene expression and antigen presentation. Synthetic biology tools now allow for rapid iteration and refinement of vaccine constructs, enabling developers to quickly adapt to evolving pathogens. CRISPR-based gene editing and computational modeling are also helping identify potent epitopes and streamline the vaccine design process. Additionally, the integration of DNA vaccines with adjuvants or heterologous prime-boost regimens (in combination with viral vectors or protein subunits) is being investigated to amplify immune responses. These innovations are bridging the gap between preclinical success and clinical effectiveness, making DNA vaccines more viable for widespread human use. With ongoing advancements in biotechnology and bioinformatics, DNA vaccines are on a trajectory to become more potent, targeted, and versatile than ever before.Why Do Regional and Sectoral Adoption Patterns Vary in the DNA Vaccines Market?
Adoption of DNA vaccines varies significantly across regions and application sectors, influenced by differences in regulatory environments, public health priorities, technological infrastructure, and disease prevalence. In North America, particularly the United States, DNA vaccine development has been strongly supported by government funding, academic research, and private biotech investment. The region has been a pioneer in clinical trials for human DNA vaccines targeting infectious diseases like Zika, HIV, and COVID-19, as well as cancers such as prostate and cervical cancer. In contrast, Asia-Pacific countries - especially India, China, and South Korea - have witnessed growing adoption in the veterinary field, where DNA vaccines are used against diseases like foot-and-mouth disease and avian influenza in livestock and poultry. Regulatory pathways for veterinary DNA vaccines are often more flexible, allowing quicker market entry compared to human applications. Europe remains cautiously optimistic, with active research but stringent regulatory hurdles that slow down market approval. Meanwhile, Latin America and Africa are emerging markets where DNA vaccines hold potential for addressing endemic diseases such as dengue, chikungunya, and malaria, though limited infrastructure and funding remain barriers. Sector-wise, veterinary applications currently dominate commercial use due to lower regulatory complexity and immediate economic benefits. However, human health applications are gaining momentum as safety, efficacy, and public acceptance continue to improve. These diverse patterns reflect the multifaceted nature of DNA vaccine adoption and the importance of regional strategies in unlocking the market's full potential.What Are the Key Drivers Propelling Growth in the DNA Vaccines Market?
The growth in the DNA vaccines market is driven by a convergence of scientific, medical, and logistical factors that underscore the technology's growing relevance in global health strategies. Foremost among these is the rising incidence of infectious diseases, emerging zoonotic threats, and viral mutations, which demand rapid, scalable, and adaptable vaccine platforms - areas where DNA vaccines excel. The COVID-19 pandemic underscored the critical need for vaccine technologies that can be quickly reprogrammed and manufactured at scale, driving public and private investment into nucleic acid platforms. DNA vaccines also offer a more favorable cold chain profile compared to mRNA vaccines, requiring only standard refrigeration, which makes them highly attractive for low- and middle-income countries seeking practical immunization solutions. Growing interest in personalized medicine is another key driver, particularly in oncology, where DNA vaccines can be tailored to individual tumor profiles for targeted immunotherapy. Additionally, rising awareness of antimicrobial resistance has renewed focus on preventative measures like vaccines, increasing market demand across both human and animal health sectors. Supportive regulatory frameworks, including fast-track designations and increased funding for biotechnology research, are further accelerating clinical development and commercialization. The proliferation of contract research and manufacturing organizations (CROs and CMOs) is enabling biotech firms to scale up quickly, while strategic collaborations between governments, NGOs, and pharmaceutical companies are driving equitable access and innovation. Together, these factors are positioning DNA vaccines as a transformative force in modern immunology and public health.Report Scope
The report analyzes the DNA Vaccines market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Oncept, West Nile-Innovator, Apex-IHN); Type (Therapeutic Vaccines, Prophylactic Vaccines); End-Use (Veterinary Clinics, Veterinary Hospitals).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Oncept segment, which is expected to reach US$445.9 Million by 2030 with a CAGR of a 5.7%. The West Nile-Innovator segment is also set to grow at 7.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $155.6 Million in 2024, and China, forecasted to grow at an impressive 6.1% CAGR to reach $137.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global DNA Vaccines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global DNA Vaccines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global DNA Vaccines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this DNA Vaccines market report include:
- AnGes, Inc.
- Astellas Pharma
- Boehringer Ingelheim GmbH
- Cadila Healthcare (Zydus Cadila)
- Eurogentec S.A.
- GeneOne Life Science, Inc.
- Geovax Biologics
- Inovio Pharmaceuticals, Inc.
- Madison Vaccines Incorporated
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Takara Bio Inc.
- Tekmira Pharmaceuticals (Arbutus)
- Thermo Fisher Scientific Inc.
- VGXI (subsidiary of GeneOne)
- Vical, Inc.
- Zydus Cadila (Cadila Healthcare)
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AnGes, Inc.
- Astellas Pharma
- Boehringer Ingelheim GmbH
- Cadila Healthcare (Zydus Cadila)
- Eurogentec S.A.
- GeneOne Life Science, Inc.
- Geovax Biologics
- Inovio Pharmaceuticals, Inc.
- Madison Vaccines Incorporated
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Takara Bio Inc.
- Tekmira Pharmaceuticals (Arbutus)
- Thermo Fisher Scientific Inc.
- VGXI (subsidiary of GeneOne)
- Vical, Inc.
- Zydus Cadila (Cadila Healthcare)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 171 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
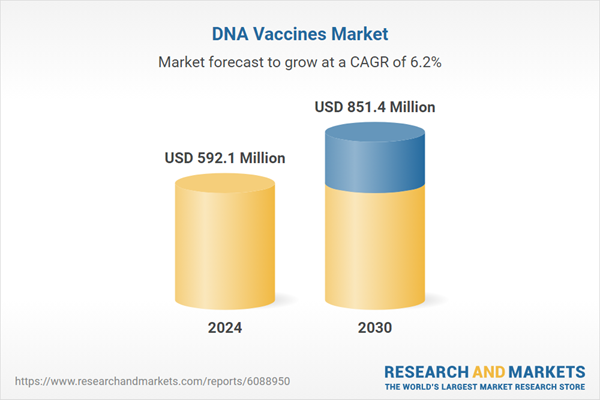
| Estimated Market Value ( USD | $ 592.1 Million |
| Forecasted Market Value ( USD | $ 851.4 Million |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |