Global Diagnostic Exosome Biomarkers Market - Key Trends & Drivers Summarized
Why Are Exosome Biomarkers Transforming the Future of Disease Diagnosis?
Exosome biomarkers are rapidly emerging as a revolutionary diagnostic tool in precision medicine, offering unprecedented insights into disease mechanisms through non-invasive liquid biopsy techniques. Exosomes are nano-sized extracellular vesicles secreted by cells into bodily fluids such as blood, saliva, urine, and cerebrospinal fluid. These vesicles carry a wealth of molecular cargo - proteins, lipids, mRNAs, microRNAs, and DNA fragments - that reflect the physiological and pathological state of their originating cells. Because exosomes circulate systemically and originate from various tissue types, including tumors and inflamed or damaged tissues, they serve as accessible, stable, and specific carriers of biomarkers for early disease detection. Their role is particularly transformative in oncology, where exosome-based diagnostics are enabling early identification of cancers such as breast, lung, prostate, and pancreatic, often before conventional imaging or tissue biopsy would reveal them. Beyond cancer, exosomal biomarkers are gaining traction in the diagnosis of neurodegenerative diseases, cardiovascular disorders, infectious diseases, and even pregnancy-related complications. As a minimally invasive diagnostic tool, exosome analysis offers significant advantages in patient compliance, repeat testing, and real-time disease monitoring, positioning it as a cornerstone in the shift toward personalized and preventive healthcare.How Are Technological Innovations Enhancing the Accuracy and Accessibility of Exosome-Based Diagnostics?
Technological advancements in isolation, purification, and characterization of exosomes are rapidly advancing the reliability and scalability of exosome biomarker diagnostics. Traditional ultracentrifugation methods, once considered the gold standard for exosome isolation, are being supplemented and replaced by faster, more efficient techniques such as microfluidics, size exclusion chromatography, immunoaffinity capture, and acoustic sorting. These technologies are improving purity and yield while reducing sample processing time, making exosome biomarker detection more compatible with clinical workflows. Analytical platforms such as nanoparticle tracking analysis (NTA), dynamic light scattering (DLS), and next-generation sequencing (NGS) are enabling high-resolution profiling of exosomal contents, while biosensors and lab-on-chip devices are bringing point-of-care exosome diagnostics closer to reality. Machine learning algorithms are also being integrated into diagnostic platforms to interpret complex exosomal data and identify disease-specific signatures with greater precision. Moreover, multiplexing technologies now allow simultaneous detection of multiple biomarkers from a single sample, increasing diagnostic power while minimizing patient discomfort. As technological integration continues, the cost of exosome analysis is expected to decline, expanding its accessibility beyond research settings into routine clinical practice. These innovations are not only driving improved diagnostic sensitivity and specificity but are also unlocking new possibilities for early detection, disease classification, and treatment stratification.Why Is the Clinical and Research Community Focusing Intensely on Exosome Biomarkers?
The growing focus on exosome biomarkers within the clinical and research communities stems from their potential to overcome limitations associated with traditional diagnostic methods. In oncology, for example, conventional tissue biopsies are invasive, sometimes infeasible, and often provide only a static snapshot of tumor biology. In contrast, exosomes offer a dynamic, longitudinal view of disease progression through routine liquid biopsies. This capability allows clinicians to track tumor evolution, monitor treatment response, and detect early signs of recurrence with greater frequency and less patient burden. In neurodegenerative conditions like Alzheimer's and Parkinson's disease, where diagnosis is often delayed or uncertain, exosomal biomarkers found in cerebrospinal fluid or plasma can reveal early pathological changes - years before clinical symptoms manifest. Similarly, in cardiovascular diseases, exosome-derived microRNAs and proteins serve as indicators of myocardial stress, vascular inflammation, and plaque instability, offering prognostic value beyond traditional lipid profiles. The research community is also exploring exosome biomarkers for their role in infectious diseases, autoimmune disorders, and organ transplant rejection. Pharmaceutical companies and academic institutions are actively collaborating to develop exosome-based diagnostic kits and integrate them into drug development pipelines. This momentum is further supported by growing regulatory interest and funding from public health bodies, underscoring the paradigm shift toward biomarker-driven diagnostics for early intervention and personalized medicine.What Are the Key Drivers Accelerating Global Growth in the Diagnostic Exosome Biomarkers Market?
The growth in the diagnostic exosome biomarkers market is being fueled by several interrelated factors spanning scientific innovation, market dynamics, regulatory evolution, and clinical demand. A primary driver is the global rise in chronic and complex diseases - particularly cancers, neurodegenerative conditions, and cardiovascular disorders - that require early, precise, and minimally invasive diagnostic approaches. Exosome biomarkers meet these needs by offering a high level of molecular specificity and the ability to detect disease at its earliest stages. Technological progress in isolation and detection tools has significantly improved the clinical viability of exosome-based tests, attracting major investments from biotechnology firms and diagnostic companies. Furthermore, the proliferation of precision medicine initiatives and the increased focus on companion diagnostics are propelling exosome biomarker adoption, as these tools can help tailor therapies based on individual molecular profiles. Regulatory bodies, including the FDA and EMA, are beginning to establish clearer pathways for biomarker validation and approval, encouraging innovation and reducing barriers to market entry. Academic-industry collaborations and multi-institutional clinical trials are expanding the evidence base for exosome diagnostics, while government-funded projects in genomics and proteomics are accelerating research. Finally, patient awareness and demand for non-invasive testing solutions are contributing to market growth, especially in outpatient and home-based care settings. Collectively, these forces are driving a robust expansion of the exosome biomarkers market, positioning it as a key player in the future of diagnostics and personalized healthcare.Report Scope
The report analyzes the Diagnostic Exosome Biomarkers market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Reagents, Kits, Serum / Plasma Kits, Urine Kits, Software); Application (Neurodegenerative Disorders, Oncology, Metabolic Disorders, Other Applications); End-Use (Cancer Institutes, Hospitals, Diagnostic Centers, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Reagents segment, which is expected to reach US$120.4 Million by 2030 with a CAGR of a 15.5%. The Kits segment is also set to grow at 21.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $33.4 Million in 2024, and China, forecasted to grow at an impressive 24.1% CAGR to reach $75.1 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Diagnostic Exosome Biomarkers Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Diagnostic Exosome Biomarkers Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Diagnostic Exosome Biomarkers Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ACS Diagnostics, AliveCor, Inc., Allengers Medical Systems, BPL Medical Technologies, CardioNet (BioTelemetry) and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Diagnostic Exosome Biomarkers market report include:
- Aethlon Medical, Inc.
- AMSBIO
- Bio-Techne Corporation
- BioVision, Inc.
- Capital Biosciences, Inc.
- Capricor Therapeutics, Inc.
- Codiak BioSciences, Inc.
- Direct Biologics LLC
- Evox Therapeutics Ltd
- Exo Biologics
- Exogenus Therapeutics
- Exosome Diagnostics, Inc.
- Exosomics S.p.A.
- Hitachi Chemical Diagnostics, Inc.
- INOVIQ Ltd
- Izon Science Ltd
- NanoFCM Inc.
- NanoSomix, Inc.
- QIAGEN N.V.
- System Biosciences, LLC
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Aethlon Medical, Inc.
- AMSBIO
- Bio-Techne Corporation
- BioVision, Inc.
- Capital Biosciences, Inc.
- Capricor Therapeutics, Inc.
- Codiak BioSciences, Inc.
- Direct Biologics LLC
- Evox Therapeutics Ltd
- Exo Biologics
- Exogenus Therapeutics
- Exosome Diagnostics, Inc.
- Exosomics S.p.A.
- Hitachi Chemical Diagnostics, Inc.
- INOVIQ Ltd
- Izon Science Ltd
- NanoFCM Inc.
- NanoSomix, Inc.
- QIAGEN N.V.
- System Biosciences, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 377 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
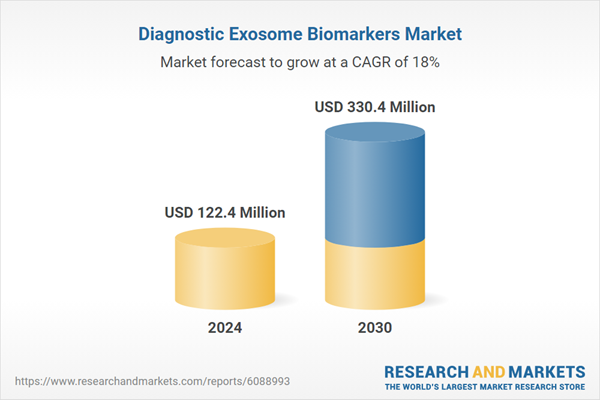
| Estimated Market Value ( USD | $ 122.4 Million |
| Forecasted Market Value ( USD | $ 330.4 Million |
| Compound Annual Growth Rate | 18.0% |
| Regions Covered | Global |