Global Demerol (Meperidine) Market - Key Trends & Drivers Summarized
Why Does Demerol (Meperidine) Remain a Notable Analgesic in Modern Medical Practice?
Demerol, also known by its generic name meperidine, remains a recognized name in the field of opioid analgesics, particularly for short-term management of moderate to severe pain. Although newer pain management alternatives have gained favor in clinical practice, Demerol continues to hold relevance in specific medical scenarios, especially in controlled environments such as emergency rooms, post-operative care units, and labor and delivery suites. Known for its rapid onset of action, meperidine is effective for managing acute pain episodes where swift analgesia is critical. It is also occasionally used as a pre-anesthetic medication and for shivering associated with anesthesia - an application where other opioids are less effective. Its continued presence in pharmacopeias across countries is due to its well-documented pharmacological profile, established manufacturing infrastructure, and familiarity among seasoned healthcare providers. However, clinicians are increasingly cautious in its use due to concerns over its active metabolite, normeperidine, which can accumulate and lead to neurotoxic effects such as seizures, especially in patients with renal impairment or in prolonged use. Despite these concerns, in acute care settings where usage is limited in scope and duration, Demerol continues to offer value as a secondary opioid when other agents are contraindicated or unavailable.How Are Shifting Prescribing Guidelines and Safety Concerns Affecting Demerol Usage?
The clinical use of Demerol (meperidine) has undergone significant scrutiny and change in recent years due to its unique risk profile and the global opioid crisis. Regulatory agencies, including the U.S. FDA and WHO, have highlighted meperidine's limitations - particularly the risks of accumulation of normeperidine, its neurotoxic metabolite, and the drug's relatively short half-life compared to other opioids. These factors have led to revised prescribing guidelines, often relegating Demerol to second- or third-line therapy in pain management protocols. Hospitals and healthcare systems are implementing stricter controls and requiring special authorization for its use, particularly in chronic care or outpatient settings. In response to this, many clinicians have shifted toward alternatives such as morphine, hydromorphone, or fentanyl, which offer more favorable pharmacokinetics and safety profiles. Nevertheless, meperidine still finds a place in highly specialized settings. It is sometimes preferred in cases of morphine allergy, or when a patient has adverse reactions to more commonly used opioids. Additionally, its historical use in palliative care has left some legacy prescribing patterns that persist in certain regions. As awareness of its risks grows, education and training on safe use, dose monitoring, and contraindications are becoming integral to ensuring Demerol's limited but responsible application in clinical practice.What Role Do Regulatory Policies and Supply Chain Dynamics Play in Market Trends?
The market for Demerol is deeply shaped by regulatory policies, manufacturing controls, and pharmaceutical supply chain dynamics, all of which collectively influence its availability and use. As a Schedule II controlled substance in the United States and similarly classified in many countries, meperidine is subject to rigorous monitoring from production through prescription, which restricts its distribution and limits unauthorized access. Regulatory efforts aimed at curbing opioid misuse have led to reductions in manufacturing quotas, tighter prescribing laws, and mandatory reporting systems, thereby compressing the overall demand and market size for meperidine. Additionally, global pharmaceutical companies have scaled back production of meperidine due to declining clinical preference and limited profit margins, sometimes resulting in supply shortages for institutions that still rely on the drug. Generic competition is minimal, and fewer manufacturers are willing to invest in maintaining production lines for aging opioids with shrinking demand. On the other hand, global health emergencies, such as pandemics or mass casualty events, may temporarily increase the need for injectable opioids, prompting governments to maintain a baseline supply of older analgesics like Demerol for emergency reserves. International regulatory harmonization and bulk purchasing agreements for essential medicines in developing countries also contribute to its continued, albeit reduced, presence in global pharmaceutical markets.What Are the Primary Factors Influencing the Future Outlook of the Meperidine Market?
The growth trajectory and future of the Demerol (meperidine) market are shaped by a confluence of medical, regulatory, and societal factors. Foremost among them is the evolution of clinical best practices, which increasingly favor newer opioids or multimodal pain management strategies that reduce opioid reliance altogether. As the healthcare industry shifts toward safer, evidence-based prescribing and embraces alternative therapies - including non-opioid analgesics, nerve blocks, and integrative pain treatments - meperidine is likely to see further declines in usage. However, its niche applications - such as treatment of post-anesthesia shivering, emergency room analgesia, and scenarios where specific patient contraindications prevent the use of other opioids - will sustain a limited but steady demand. The increasing digitization of healthcare records and electronic prescribing is also playing a role by enabling better oversight of opioid use, minimizing off-label or extended prescriptions of high-risk drugs like meperidine. Additionally, growing public pressure to address the opioid epidemic and expand access to addiction treatment is driving a regulatory environment that is cautious about older opioids' role in modern medicine. Yet, global disparities in healthcare infrastructure, particularly in low- and middle-income countries, may sustain meperidine's relevance in settings with limited access to a broader pharmacological arsenal. Overall, while the Demerol market is unlikely to grow significantly, it will continue to serve specialized roles, maintained by stringent oversight and informed clinical use.Report Scope
The report analyzes the Demerol Meperdine market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Parenteral, Tablets); Application (Pain Relief, Anesthesia, Cough Suppression, Diarrhea Suppression, De-Addiction).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Parenteral Adminstration segment, which is expected to reach US$244.8 Million by 2030 with a CAGR of a 1.3%. The Tablets Adminstration segment is also set to grow at 2.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $88 Million in 2024, and China, forecasted to grow at an impressive 3.3% CAGR to reach $66 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Demerol Meperdine Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Demerol Meperdine Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Demerol Meperdine Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Acuity Brands Lighting, Inc., Articolo Studios, Brightech, Cooper Lighting Solutions, Crest LED Lighting and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Demerol Meperdine market report include:
- Actavis PLC
- Boehringer Ingelheim
- Cantrell Drug Company
- Curia Global, Inc.
- Egalet Corporation
- Endo Pharmaceuticals Inc.
- Epic Pharma, LLC
- Genus Lifesciences Inc.
- Hikma Pharmaceuticals USA
- Hospira, Inc.
- Janssen Pharmaceuticals, Inc.
- Mallinckrodt Pharmaceuticals
- Pfizer Inc.
- Physicians Total Care, Inc.
- PRG Pharma Pvt. Ltd.
- Saneca Pharmaceuticals
- Sanofi
- Sun Pharmaceutical Industries Ltd.
- Tenatra Chemie
- Vintage Pharmaceuticals, LLC
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Actavis PLC
- Boehringer Ingelheim
- Cantrell Drug Company
- Curia Global, Inc.
- Egalet Corporation
- Endo Pharmaceuticals Inc.
- Epic Pharma, LLC
- Genus Lifesciences Inc.
- Hikma Pharmaceuticals USA
- Hospira, Inc.
- Janssen Pharmaceuticals, Inc.
- Mallinckrodt Pharmaceuticals
- Pfizer Inc.
- Physicians Total Care, Inc.
- PRG Pharma Pvt. Ltd.
- Saneca Pharmaceuticals
- Sanofi
- Sun Pharmaceutical Industries Ltd.
- Tenatra Chemie
- Vintage Pharmaceuticals, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 287 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
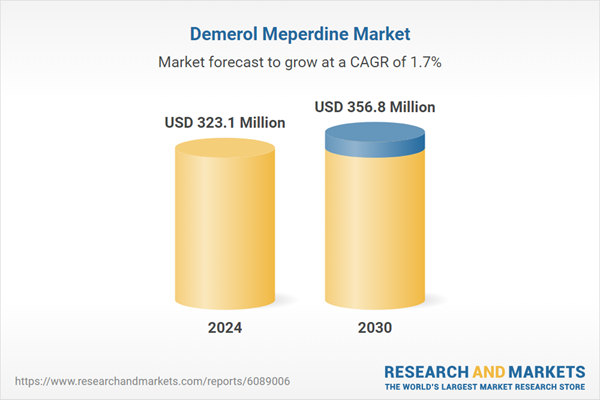
| Estimated Market Value ( USD | $ 323.1 Million |
| Forecasted Market Value ( USD | $ 356.8 Million |
| Compound Annual Growth Rate | 1.7% |
| Regions Covered | Global |