Global CRISPR Genomic Cure Market - Key Trends & Drivers Summarized
Why Is CRISPR Emerging as a Groundbreaking Platform for Curative Genomic Medicine?
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology is revolutionizing the field of medicine by offering a powerful and precise tool for editing genes and potentially curing previously untreatable genetic disorders. Unlike traditional therapies that manage symptoms, CRISPR-based genomic cures target the root cause of disease at the DNA level, making them fundamentally transformative. At its core, CRISPR allows scientists to “cut and paste” sections of DNA with unprecedented accuracy, enabling the correction of mutations responsible for a wide range of conditions - from monogenic diseases like sickle cell anemia and cystic fibrosis to more complex disorders including cancer and certain neurodegenerative diseases. The therapeutic potential of CRISPR has sparked a wave of innovation and investment across biotech and pharmaceutical sectors, with several therapies entering clinical trials and some showing promising early results. The simplicity, cost-effectiveness, and versatility of CRISPR compared to earlier gene-editing methods such as TALENs or zinc finger nucleases have accelerated its adoption in both research and clinical settings. Additionally, advancements like CRISPR-Cas9, base editing, and prime editing are further expanding its therapeutic possibilities. As the medical community shifts its focus toward precision medicine and curative treatments, CRISPR stands at the forefront holding promise for eradicating inherited diseases and transforming the future of healthcare.How Are Clinical Trials, Regulatory Evolution, and Biotech Innovation Driving CRISPR Therapies Forward?
The global advancement of CRISPR-based genomic cures is being propelled by a growing number of clinical trials, progressive regulatory frameworks, and robust innovation within the biotechnology industry. Clinical trials are currently underway for a variety of applications, including blood disorders such as beta-thalassemia and sickle cell disease, where initial results have shown significant improvements in patient outcomes following a single treatment. Regulatory bodies like the U.S. FDA and the European Medicines Agency are adapting their evaluation criteria to accommodate the complexities of gene-editing therapies, issuing specific guidelines for genome-modifying technologies and expediting the approval process through designations like Orphan Drug, Fast Track, and Breakthrough Therapy. This regulatory openness is fostering a more favorable environment for companies to bring CRISPR treatments to market. Meanwhile, biotech startups and pharmaceutical giants are forming partnerships to combine scientific expertise, intellectual property, and clinical infrastructure to accelerate development. The creation of delivery systems - such as viral vectors and lipid nanoparticles - that ensure precise and safe transport of CRISPR components to target cells is a major area of innovation. Moreover, developments in off-target effect detection and mitigation are increasing the safety profile of CRISPR therapies. With a growing ecosystem of investors, researchers, and regulators working collaboratively, the clinical and commercial pathway for CRISPR genomic cures is becoming clearer and more achievable.What Ethical, Social, and Technical Considerations Are Shaping the Global CRISPR Landscape?
As CRISPR moves closer to clinical mainstream adoption, ethical, social, and technical considerations are becoming increasingly critical to the technology's responsible development and deployment. One of the most prominent ethical concerns revolves around germline editing - changes to embryos or reproductive cells that can be passed on to future generations. While current therapeutic applications are largely confined to somatic cell editing (non-inheritable), public discourse and regulatory policies continue to wrestle with the implications of permanent genomic alterations. Questions of equity and access also dominate discussions: Will CRISPR therapies be affordable and available to all, or only to wealthy patients in developed countries? These concerns are driving calls for global governance frameworks and bioethics oversight that ensure transparency, accountability, and inclusivity. Technically, issues such as off-target effects, incomplete edits, immune responses, and long-term safety remain focal points of ongoing research. Scientists are also working on refining CRISPR systems to reduce unintended genetic changes and improve targeting specificity. At the societal level, patient advocacy groups, public awareness campaigns, and stakeholder engagement initiatives are playing a vital role in shaping perceptions and policies. Additionally, cultural and religious attitudes toward genetic modification vary widely, adding complexity to global adoption. These intertwined challenges underscore the need for interdisciplinary collaboration among scientists, ethicists, policymakers, and communities to harness CRISPR's full potential in an ethical and equitable manner.What Are the Key Drivers Fueling the Growth of the CRISPR Genomic Cure Market Worldwide?
The growth in the CRISPR genomic cure market is driven by a confluence of scientific breakthroughs, increasing prevalence of genetic disorders, supportive regulatory environments, and rising investments from public and private sectors. A major driver is the rising global burden of genetic diseases - both rare and common - which remain poorly managed or incurable through conventional therapies. CRISPR's ability to offer a one-time, potentially permanent solution to these conditions is a major draw for healthcare systems aiming to reduce long-term treatment costs and improve patient outcomes. The sharp increase in genomics research funding and CRISPR-related patents has led to a surge in pipeline development across biotech firms, academic institutions, and contract research organizations. Additionally, growing public and institutional acceptance of gene-editing as a valid therapeutic approach is encouraging more clinical trials and cross-border collaborations. Technological improvements in guide RNA design, delivery vectors, and gene-editing enzymes are lowering development barriers and enhancing therapeutic efficacy. Meanwhile, the integration of CRISPR with AI, big data analytics, and personalized medicine platforms is enabling more accurate patient stratification and treatment planning. Health policy initiatives aimed at supporting rare disease research, combined with patient advocacy, are further pushing CRISPR into regulatory fast lanes. Together, these drivers are catalyzing a paradigm shift in the life sciences sector, where curative genomic therapies are not only a scientific aspiration but an imminent reality poised to redefine the future of medicine.Report Scope
The report analyzes the CRISPR Genomic Cure market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Kits & Reagents, Libraries, Vector Design, Assay Design, Other Product Types); Application (Oncology, Genetic Diseases, Genomic Engineering, Other Applications); End-User (Biopharmaceutical Companies, Contract Research Organizations, Genetic Research Laboratories, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Kits & Reagents segment, which is expected to reach US$5.8 Billion by 2030 with a CAGR of a 23.3%. The Libraries segment is also set to grow at 22.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1 Billion in 2024, and China, forecasted to grow at an impressive 28.3% CAGR to reach $2.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global CRISPR Genomic Cure Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global CRISPR Genomic Cure Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global CRISPR Genomic Cure Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Credit Benchmark, CreditRiskMonitor, CreditVidya, CRISIL Limited, Dun & Bradstreet and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this CRISPR Genomic Cure market report include:
- Beam Therapeutics
- Caribou Biosciences
- CRISPR Biotech Engineering
- CRISPR Therapeutics
- Editas Medicine
- ERS Genomics
- GenScript
- Horizon Discovery
- Inscripta
- Intellia Therapeutics
- Mammoth Biosciences
- Prime Medicine
- Scribe Therapeutics
- Synthego
- Takara Bio
- Thermo Fisher Scientific
- ToolGen
- Vertex Pharmaceuticals
- Verve Therapeutics
- Wave Life Sciences
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Beam Therapeutics
- Caribou Biosciences
- CRISPR Biotech Engineering
- CRISPR Therapeutics
- Editas Medicine
- ERS Genomics
- GenScript
- Horizon Discovery
- Inscripta
- Intellia Therapeutics
- Mammoth Biosciences
- Prime Medicine
- Scribe Therapeutics
- Synthego
- Takara Bio
- Thermo Fisher Scientific
- ToolGen
- Vertex Pharmaceuticals
- Verve Therapeutics
- Wave Life Sciences
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 282 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
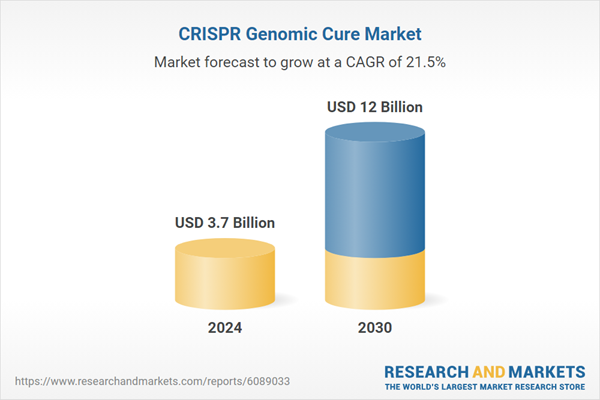
| Estimated Market Value ( USD | $ 3.7 Billion |
| Forecasted Market Value ( USD | $ 12 Billion |
| Compound Annual Growth Rate | 21.5% |
| Regions Covered | Global |