Global Blood Banking Devices Market - Key Trends & Drivers Summarized
Why Are Blood Banking Devices Gaining Strategic Significance in Transfusion Medicine, Public Health Preparedness, and Clinical Supply Chain Optimization?
Blood banking devices are essential for the safe collection, processing, storage, and distribution of blood and its components, forming the operational backbone of transfusion services and emergency medical response systems. As global demand for blood products rises - driven by trauma care, surgical interventions, cancer therapies, and hematological disorders - health systems are increasingly prioritizing automation, standardization, and traceability within blood bank workflows. Devices such as blood collection monitors, centrifuges, leukoreduction filters, component separators, and automated storage systems are critical in maintaining product quality, minimizing wastage, and ensuring donor-to-recipient safety.The strategic importance of blood banking infrastructure has been reinforced by recent global health emergencies, where rapid mobilization of blood supplies and robust donor management systems have proven essential. Devices that enable faster, sterile, and high-yield processing of blood components support public health resiliency, particularly in low-resource settings or during outbreaks, natural disasters, and mass casualty scenarios. Moreover, the growing emphasis on patient blood management (PBM) is increasing reliance on precise and consistent processing technologies to reduce unnecessary transfusions and improve clinical outcomes.
As healthcare moves toward centralized laboratory services and integrated care models, blood banking devices are becoming part of larger diagnostic and therapeutic ecosystems. Their role extends beyond logistics and storage to include data interoperability, compliance assurance, and quality control - making them integral to value-based care delivery in hospitals, blood centers, and national transfusion networks.
How Are Automation, Cold Chain Innovation, and Digital Integration Enhancing Efficiency and Reliability in Blood Banking?
Automation is reshaping blood bank operations by reducing manual error, increasing throughput, and standardizing process parameters. Automated blood component separators, plasma extractors, and tube sealers are enabling high-volume, high-precision processing of whole blood into red cells, plasma, and platelets. These systems are essential for meeting stringent regulatory standards on sterility, yield, and component separation timelines, particularly in high-demand or multi-shift operational settings.Advanced refrigeration and cold chain devices - such as temperature-monitored blood bank refrigerators, platelet incubators, and plasma freezers - are ensuring temperature integrity and compliance with AABB, FDA, and WHO guidelines. Real-time temperature logging, deviation alerts, and remote monitoring capabilities reduce product loss and enhance audit readiness. Additionally, solar-powered and battery-backed cold storage systems are extending access to reliable blood product storage in off-grid or mobile environments.
Digital integration is enabling end-to-end visibility across blood banking workflows. Integration with Laboratory Information Systems (LIS), inventory tracking modules, and RFID-enabled labeling systems supports donor traceability, inventory optimization, and transfusion compatibility checks. Cloud-based platforms are allowing centralized oversight of multi-site operations, while AI and predictive analytics are beginning to play a role in forecasting blood demand, optimizing donor outreach, and minimizing shelf-life expiration across regional networks.
Which Healthcare Systems, End-Use Segments, and Regional Markets Are Driving Adoption of Blood Banking Devices?
Hospitals, standalone blood banks, military field units, and national transfusion services are the primary adopters of blood banking devices. Large academic medical centers and trauma facilities require integrated systems for on-demand component preparation and transfusion safety, while mobile collection units and rural health programs prioritize compact, rugged, and automated solutions suitable for decentralized settings. The expansion of public-private blood bank partnerships and voluntary donor programs is further amplifying device demand across both government and NGO-driven networks.North America and Western Europe dominate the market in terms of technological sophistication, regulatory compliance, and infrastructure density. However, Asia-Pacific and parts of Latin America are experiencing the fastest growth, fueled by rising surgical volumes, maternal and neonatal care demands, and improving health system financing. Countries like India, China, Brazil, and Indonesia are scaling up national blood services through donor education, improved screening, and expanded cold chain logistics, driving significant procurement of advanced blood banking equipment.
Procurement models are increasingly focused on lifecycle value, encompassing equipment performance, service uptime, regulatory documentation, and training support. Turnkey packages including hardware, software, and after-sales service are gaining favor, particularly in multi-site networks and public health systems. As procurement priorities evolve toward total cost of ownership and clinical integration, suppliers offering scalable, digitally enabled, and standards-compliant solutions are gaining competitive traction.
What Are the Factors Driving Growth in the Blood Banking Devices Market?
The blood banking devices market is expanding as healthcare systems prioritize safe, efficient, and traceable transfusion workflows in response to rising procedural volumes, chronic disease burdens, and public health preparedness mandates. These devices are enabling higher product yield, enhanced safety, and streamlined operations across donor-to-recipient pathways.Key growth drivers include the rise in surgical and trauma-related transfusions, increased automation in blood processing, digital integration of donor and inventory data, and growing investment in transfusion infrastructure across emerging markets. Enhanced compliance requirements and global initiatives in blood safety are further catalyzing technology adoption.
As precision in transfusion medicine becomes inseparable from operational resilience and patient safety, could blood banking devices emerge as critical enablers of a more responsive, digitized, and globally connected blood supply ecosystem?
Report Scope
The report analyzes the Blood Banking Devices market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Blood Collection Devices, Blood Processing Devices, Blood Storage Devices, Blood Transfusion Devices, Blood Grouping / Testing Reagents, Other Product Types); Collection Method (Manual Blood Collection, Automated Blood Collection); End-User (Hospitals, Clinics, Blood Banks, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Blood Collection Devices segment, which is expected to reach US$8.9 Billion by 2030 with a CAGR of a 7.3%. The Blood Processing Devices segment is also set to grow at 4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $6.3 Billion in 2024, and China, forecasted to grow at an impressive 9% CAGR to reach $6.5 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Blood Banking Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Blood Banking Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Blood Banking Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alfred Miller Contracting, Anchor Modular Buildings, ATCO Structures & Logistics, BOXX Modular, Bulletproof Building Ltd and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Blood Banking Devices market report include:
- Abbott Laboratories
- Beckman Coulter, Inc.
- Becton, Dickinson and Company (BD)
- Bio-Rad Laboratories, Inc.
- Cardinal Health
- Cerus Corporation
- China Biologic Products Holdings, Inc.
- Fresenius Kabi AG
- Grifols, S.A.
- Haemonetics Corporation
- Hindustan Syringes & Medical Devices Ltd.
- Immucor, Inc.
- Macopharma
- Ortho Clinical Diagnostics
- Polymed Medical Devices
- Roche Diagnostics
- Sartorius AG
- Siemens Healthineers AG
- Terumo Corporation
- Thermo Fisher Scientific Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Beckman Coulter, Inc.
- Becton, Dickinson and Company (BD)
- Bio-Rad Laboratories, Inc.
- Cardinal Health
- Cerus Corporation
- China Biologic Products Holdings, Inc.
- Fresenius Kabi AG
- Grifols, S.A.
- Haemonetics Corporation
- Hindustan Syringes & Medical Devices Ltd.
- Immucor, Inc.
- Macopharma
- Ortho Clinical Diagnostics
- Polymed Medical Devices
- Roche Diagnostics
- Sartorius AG
- Siemens Healthineers AG
- Terumo Corporation
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 384 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
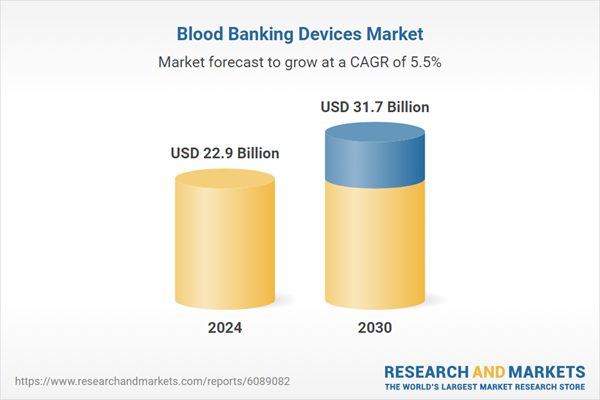
| Estimated Market Value ( USD | $ 22.9 Billion |
| Forecasted Market Value ( USD | $ 31.7 Billion |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |