Global Blarcamesine Market - Key Trends & Drivers Summarized
Why Is Blarcamesine Gaining Prominence as a Novel Therapeutic Candidate in the Treatment of Neurodegenerative and Neurodevelopmental Disorders?
Blarcamesine (also known as ANAVEX®2-73) is emerging as a clinically significant small molecule drug candidate targeting central nervous system (CNS) disorders, particularly Alzheimer's disease, Rett syndrome, and Parkinson's disease dementia. Designed to modulate the sigma-1 receptor (S1R) and muscarinic receptors, Blarcamesine works through a multi-modal mechanism of action that supports cellular homeostasis, synaptic plasticity, and mitochondrial function - key pathways implicated in neurodegeneration and neuroinflammation. Its pharmacological profile positions it as a first-in-class agent capable of addressing both symptomatic relief and underlying disease modification.Unlike many CNS therapies that target singular pathological markers, Blarcamesine's S1R activation offers a system-level approach that may impact a broader range of neurological pathways, including calcium homeostasis, endoplasmic reticulum stress response, and oxidative damage. Clinical trial data to date have indicated potential cognitive, behavioral, and functional improvements in target populations, alongside favorable safety and tolerability metrics. These multi-dimensional effects support Blarcamesine's potential as a versatile therapeutic across both rare and prevalent neurodegenerative diseases, particularly those with limited existing treatment options.
The growing unmet need for effective CNS therapeutics - compounded by an aging global population and rising incidence of Alzheimer's and Parkinsonian syndromes - is accelerating interest in novel mechanisms such as sigma-1 modulation. Blarcamesine's orphan drug status in Rett syndrome and Fast Track designation by the FDA for Alzheimer's disease further underscore its strategic potential. As neuropharmaceutical pipelines pivot toward disease-modifying agents, Blarcamesine stands out for its dual impact on symptom control and neuroprotective activity.
How Are Clinical Developments, Regulatory Pathways, and Biomarker-Based Patient Stratification Supporting Blarcamesine's Market Trajectory?
Progress in Blarcamesine's clinical development program is a key driver of its commercialization potential. Ongoing Phase II and III studies across Alzheimer's disease and Rett syndrome are evaluating its efficacy in improving cognitive function, seizure activity, and quality-of-life metrics. Interim data have shown statistically significant responses in pre-specified subpopulations, highlighting the importance of precision medicine approaches in CNS drug development. In particular, the use of genomic and protein biomarkers - such as SIGMAR1 expression and APOE genotype - has enabled more targeted patient selection and enhanced therapeutic relevance.The compound's regulatory momentum is supported by multiple designations, including orphan drug, Fast Track, and rare pediatric disease designations, which can expedite review timelines, support market exclusivity, and de-risk development. These regulatory advantages are particularly critical in rare disease segments like Rett syndrome, where rapid development and approval pathways can accelerate time to market. In parallel, the expansion of patient registries and natural history studies is supporting real-world evidence generation, a key asset for both regulatory engagement and reimbursement discussions.
Anavex Life Sciences, the sponsor of Blarcamesine, is also investing in digital biomarker development and artificial intelligence-driven analytics to refine responder identification and optimize trial endpoints. These strategies are enhancing data granularity and enabling a more predictive understanding of treatment response, potentially lowering the risk of late-stage failure. As CNS trials increasingly adopt decentralized and adaptive designs, Blarcamesine's development model reflects broader industry trends toward precision neuroscience and patient-centric evaluation frameworks.
Which Indications, Market Access Dynamics, and Strategic Partnerships Are Driving Commercial Outlook for Blarcamesine?
Blarcamesine's primary indications - Alzheimer's disease, Rett syndrome, and Parkinson's disease dementia - represent distinct but high-value segments within the neurodegenerative and neurodevelopmental therapeutic landscape. Alzheimer's remains the largest commercial opportunity, given its global prevalence, limited treatment arsenal, and substantial economic burden. Rett syndrome, though rare, offers a fast-track pathway to market via orphan drug incentives and serves as a strategic beachhead for broader CNS portfolio expansion. Parkinson's disease dementia represents an additional growth lever, with Blarcamesine positioned to address cognitive and motor symptom overlap.Market access will hinge on demonstrating clinically meaningful outcomes in cognition, behavior, and quality of life, supported by pharmacoeconomic modeling and real-world data integration. As payers demand evidence of disease-modifying effects and value-based outcomes, Blarcamesine's ability to show multi-domain efficacy will be critical. Patient advocacy, early engagement with HTA bodies, and publication of peer-reviewed efficacy and safety data are expected to play central roles in shaping reimbursement and formulary positioning, especially in publicly funded health systems.
Strategic partnerships - whether with larger pharmaceutical companies, academic consortia, or biomarker platform providers - will be instrumental in scaling development, expanding geographic reach, and navigating post-approval commercialization. Licensing, co-development, or distribution agreements may be pursued to optimize launch in key regions, particularly where rare disease infrastructure or CNS specialist networks are already in place. As CNS drug development regains investor and industry focus, Blarcamesine's differentiated mechanism and promising data trajectory position it as a potential frontrunner in reshaping treatment paradigms for complex brain disorders.
What Are the Factors Driving Growth in the Blarcamesine Market?
The Blarcamesine market is advancing as neuroscience research shifts toward multi-target, biomarker-guided therapies capable of addressing both functional symptoms and neurobiological disease progression. Its S1R-targeting profile offers a differentiated therapeutic mechanism across high-unmet-need CNS conditions.Key growth drivers include positive clinical momentum, orphan and Fast Track regulatory designations, precision-medicine-based patient selection, and growing demand for disease-modifying treatments in neurodegeneration and rare CNS disorders. The increasing emphasis on non-dopaminergic, non-amyloid pathways in CNS drug development further amplifies its relevance.
As neuropharmaceutical innovation redefines therapeutic possibilities, could Blarcamesine represent a new benchmark in multi-modal CNS intervention - delivering functional restoration through a mechanism that bridges neuroplasticity, cellular resilience, and precision medicine?
Report Scope
The report analyzes the Blarcamesine market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Administration Route (Oral, Parenteral); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies); Application (Alzheimer's Disease, Parkinson's Disease, Dementia, Rett Syndrome, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Oral Administration segment, which is expected to reach US$596.6 Million by 2030 with a CAGR of a 1.7%. The Parenteral Administration segment is also set to grow at 3.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $209.1 Million in 2024, and China, forecasted to grow at an impressive 4.3% CAGR to reach $166.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Blarcamesine Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Blarcamesine Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Blarcamesine Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, AdvanCell, AstraZeneca, Bristol-Myers Squibb Company, Celgene Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Blarcamesine market report include:
- AbbVie Inc.
- Amgen Inc.
- Anavex Life Sciences Corp.
- AstraZeneca
- Biogen Inc.
- Bristol-Myers Squibb
- Eli Lilly and Co.
- GlaxoSmithKline
- Johnson & Johnson
- Merck & Co.
- Novartis
- Pfizer
- Roche Holding AG
- Sanofi
- Teva Pharmaceutical Industries Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Amgen Inc.
- Anavex Life Sciences Corp.
- AstraZeneca
- Biogen Inc.
- Bristol-Myers Squibb
- Eli Lilly and Co.
- GlaxoSmithKline
- Johnson & Johnson
- Merck & Co.
- Novartis
- Pfizer
- Roche Holding AG
- Sanofi
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 370 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
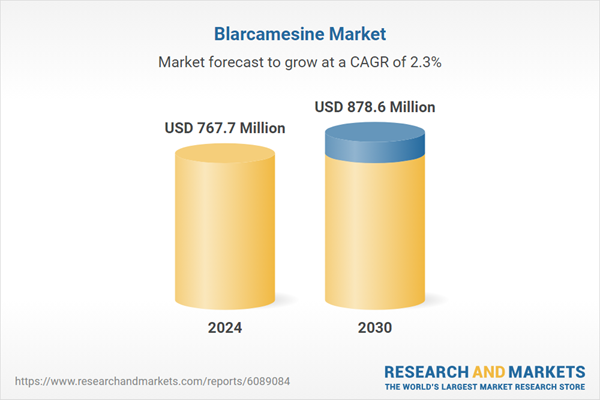
| Estimated Market Value ( USD | $ 767.7 Million |
| Forecasted Market Value ( USD | $ 878.6 Million |
| Compound Annual Growth Rate | 2.3% |
| Regions Covered | Global |