Global Bladder Cancer Detective Kits Market - Key Trends & Drivers Summarized
Why Are Bladder Cancer Detective Kits Gaining Strategic Relevance in Early Diagnosis, Recurrence Monitoring, and Non-Invasive Oncology Workflows?
Bladder cancer detective kits are becoming vital tools in the diagnostic and post-treatment surveillance landscape due to the high recurrence rate, asymptomatic progression, and need for long-term monitoring associated with bladder malignancies. These kits enable early detection of urothelial carcinoma markers through non-invasive means - primarily urine-based assays - offering an alternative to more invasive and costly diagnostic procedures such as cystoscopy. As precision medicine and patient-centric care models gain ground, these kits are increasingly viewed as enablers of efficient, scalable, and accessible oncology workflows.With bladder cancer ranking among the most recurrent malignancies globally, there is a strong clinical imperative for diagnostic tools that can reliably track disease status across time without compromising patient comfort. Urine-based detective kits - utilizing immunoassay, PCR, FISH, or ELISA technologies - detect tumor-associated biomarkers such as NMP22, BTA, and cytokeratin fragments, supporting both initial diagnosis and recurrence surveillance. These kits are especially valuable for high-risk patients, the elderly, and those under routine follow-up, offering quicker turnaround and reduced healthcare burden.
The ability of bladder cancer detective kits to be deployed in outpatient, primary care, and remote testing environments positions them as cost-effective diagnostic adjuncts, especially in resource-limited settings. Their non-invasive nature enhances patient compliance, facilitates longitudinal monitoring, and allows for more frequent screening - potentially reducing the clinical and economic impact of late-stage disease progression. These attributes are reinforcing their strategic role in early intervention, risk stratification, and patient retention across urology and oncology practices.
How Are Biomarker Advancements, Multiplexing Technologies, and Point-of-Care Platforms Enhancing Diagnostic Accuracy and Market Differentiation?
Ongoing advancements in biomarker discovery and validation are driving the sensitivity and specificity of bladder cancer detection kits. Multi-marker panels that combine DNA methylation profiles, RNA signatures, exosomal content, and protein biomarkers are improving diagnostic confidence and reducing false positives. Integration of next-generation sequencing (NGS) and digital PCR is allowing kits to detect early mutational events and minimal residual disease, which is particularly valuable for surveillance in post-surgical and BCG-treated patients.Multiplexing technologies are enabling simultaneous detection of multiple biomarkers from a single urine sample, enhancing diagnostic efficiency and enabling broader clinical application within a streamlined workflow. These innovations support differentiation among kit manufacturers by improving diagnostic yield without increasing sample volume or turnaround time. Advanced signal amplification, microfluidic assay platforms, and machine learning-based interpretation tools are further improving performance metrics, particularly in detecting low-grade and early-stage bladder cancers.
Point-of-care diagnostic kits are also gaining traction, especially in ambulatory settings and community health programs. Portable devices integrated with lateral flow assays, smartphone-enabled readers, or lab-on-chip formats are enabling rapid testing outside traditional lab infrastructure. These platforms are particularly relevant in emerging markets, rural screening initiatives, and outpatient follow-up programs, where accessibility and affordability are paramount. As diagnostics move closer to the patient, ease-of-use and clinical integration are becoming key competitive differentiators in the bladder cancer detective kit space.
Which Clinical Segments, Regional Health Systems, and Adoption Pathways Are Driving Market Expansion for Bladder Cancer Detective Kits?
Surveillance and high-risk screening constitute the largest application segments, as the majority of bladder cancer patients require lifelong monitoring for recurrence. Kits are increasingly being integrated into post-cystectomy follow-up protocols, BCG therapy response monitoring, and urology outpatient workflows. Adjunctive use alongside cystoscopy is also rising, particularly in patients for whom invasive diagnostics are impractical or poorly tolerated, such as the elderly or comorbid populations.North America and Western Europe lead in adoption due to well-established cancer screening infrastructure, high awareness among urologists, and favorable reimbursement frameworks for diagnostic testing. Asia-Pacific is emerging as a high-growth region, driven by expanding urology services, increased cancer incidence, and rising investment in decentralized diagnostic infrastructure. Countries such as China, India, and Japan are witnessing heightened activity in early detection campaigns and domestic diagnostic innovation. In low- and middle-income economies, international funding and public-private partnerships are supporting the inclusion of non-invasive kits in national cancer screening initiatives.
Hospital laboratories, diagnostic chains, and point-of-care providers are key customer segments, alongside reference labs and specialty urology clinics. Market access strategies are increasingly focused on regulatory approvals, diagnostic guideline inclusion, and payer alignment, with companion diagnostic partnerships also gaining ground - especially for kits targeting therapeutic eligibility (e.g., PD-L1 or FGFR testing). As competitive intensity rises, success is being defined by clinical validation strength, platform integration capability, and alignment with evolving cancer care delivery models.
What Are the Factors Driving Growth in the Bladder Cancer Detective Kits Market?
The bladder cancer detective kits market is expanding rapidly as healthcare systems seek efficient, patient-friendly tools to address the high recurrence burden and diagnostic complexity of urothelial malignancies. These kits are enabling earlier detection, improved surveillance, and enhanced care continuity across urology and oncology pathways.Key growth drivers include rising bladder cancer incidence, growing emphasis on non-invasive and outpatient diagnostics, biomarker-driven innovation, and expansion of screening programs in both developed and emerging markets. Advances in assay sensitivity, multiplexing capabilities, and decentralized testing are further propelling adoption.
As precision diagnostics converge with personalized surveillance models, could bladder cancer detective kits become foundational tools in reshaping how healthcare systems detect, monitor, and manage one of the most persistent cancers in modern oncology?
Report Scope
The report analyzes the Bladder Cancer Detective Kits market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Technology (Fluorescence In Situ Hybridization, Enzyme-Linked Immunosorbent Assay, Other Technologies); End-Use (Hospitals & Clinics, Diagnostic Centers, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Fluorescence In Situ Hybridization Technology segment, which is expected to reach US$235.7 Million by 2030 with a CAGR of a 12.9%. The Enzyme-Linked Immunosorbent Assay Technology segment is also set to grow at 8.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $54.1 Million in 2024, and China, forecasted to grow at an impressive 15.5% CAGR to reach $79.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Bladder Cancer Detective Kits Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Bladder Cancer Detective Kits Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Bladder Cancer Detective Kits Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ABB Ltd., Amphenol Corporation, Cobo Group, Crydom (Sensata Technologies), Eaton Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Bladder Cancer Detective Kits market report include:
- Abbott Laboratories
- Abingdon Health
- Alfa Scientific Designs
- Ameritek, Inc.
- Celnovte Biotechnology Co., Ltd.
- Cepheid
- Diagnosis S.A.
- Exact Sciences
- Hubei Jinjian Biology
- KDx Diagnostics Inc.
- Nanjing Liming Bio-products Co., Ltd.
- NanoEnTek Inc.
- Nonacus Ltd.
- Nucleix Ltd.
- Pacific Edge Limited
- Pangea Laboratory
- Photocure ASA
- Polymedco Cancer Diagnostic
- Shanghai Epiprobe Biotechnology Co., Ltd.
- Xiamen Biotime Biotechnology Co., Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Abingdon Health
- Alfa Scientific Designs
- Ameritek, Inc.
- Celnovte Biotechnology Co., Ltd.
- Cepheid
- Diagnosis S.A.
- Exact Sciences
- Hubei Jinjian Biology
- KDx Diagnostics Inc.
- Nanjing Liming Bio-products Co., Ltd.
- NanoEnTek Inc.
- Nonacus Ltd.
- Nucleix Ltd.
- Pacific Edge Limited
- Pangea Laboratory
- Photocure ASA
- Polymedco Cancer Diagnostic
- Shanghai Epiprobe Biotechnology Co., Ltd.
- Xiamen Biotime Biotechnology Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 271 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
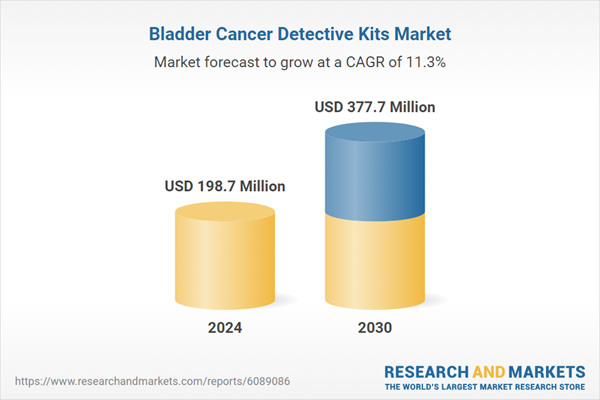
| Estimated Market Value ( USD | $ 198.7 Million |
| Forecasted Market Value ( USD | $ 377.7 Million |
| Compound Annual Growth Rate | 11.3% |
| Regions Covered | Global |