Global Biopharma Buffer Market - Key Trends & Drivers Summarized
Why Are Buffers Gaining Strategic Importance Across Biopharmaceutical Manufacturing, Biologic Stability, and Process Consistency?
Buffers are critical process components in biopharmaceutical production, serving essential roles in maintaining pH stability, ensuring protein conformation, and safeguarding the structural integrity of biologics across upstream, downstream, and fill-finish stages. As the complexity and sensitivity of therapeutic proteins, monoclonal antibodies, and cell- and gene-based therapies increase, the demand for high-purity, GMP-grade buffer systems has risen sharply. Biopharma buffers are now seen as process enablers - integral to achieving product consistency, regulatory compliance, and high-yield manufacturing efficiency.The expansion of biologics pipelines and the rise of modality-diverse platforms - such as mRNA, ADCs, and viral vectors - have elevated the importance of tailor-made buffer formulations optimized for molecule-specific stability, solubility, and purification compatibility. Buffers are used extensively in cell culture media preparation, chromatography, viral inactivation, ultrafiltration, and lyophilization processes, requiring precision control over ionic strength, osmolality, and sterility. Their role in mitigating aggregation, degradation, and pH drift is especially critical in maintaining quality attributes across extended production cycles and distributed manufacturing networks.
As the industry moves toward intensified and continuous bioprocessing models, buffers must perform consistently across dynamic flow conditions, high-throughput formats, and automated platforms. Single-use buffer systems, buffer concentrates, and on-demand buffer preparation technologies are gaining traction as manufacturers seek to reduce footprint, simplify logistics, and increase operational agility. The strategic shift from commoditized raw materials to performance-critical inputs is redefining the role of buffers in modern biomanufacturing ecosystems.
How Are Formulation Innovation, Supply Chain Optimization, and Automation Enhancing Buffer Functionality and Scalability?
Formulation innovation is a key focus area, with manufacturers developing multi-component buffer systems tailored to specific bioprocess phases and molecule characteristics. High-concentration buffer solutions, pre-blended stock formulations, and low-endotoxin, low-metal variants are being optimized to minimize contamination risk, reduce preparation time, and ensure reproducibility. pH and conductivity precision are being tightened to align with QbD (Quality by Design) and PAT (Process Analytical Technology) frameworks across upstream expression and downstream purification.Supply chain efficiency is also driving demand for pre-packaged, ready-to-use buffer systems that eliminate in-house weighing, mixing, and filtration steps. Bulk liquid delivery, flexible container systems, and modular storage solutions are reducing preparation errors and improving compliance in cGMP environments. Partnerships between buffer suppliers and biomanufacturers are becoming more collaborative, with co-development of buffer platforms aligned to biosimilar manufacturing or site-specific process platforms.
Automation and digitalization are accelerating buffer management, with buffer preparation modules integrated into automated skids and single-use bioreactor platforms. Real-time monitoring tools track buffer consumption, inventory levels, and process parameters, supporting closed-loop control and predictive resupply. Integrated LIMS, MES, and digital batch records are reinforcing traceability and audit readiness, particularly for contract manufacturing organizations (CMOs) and multi-site bioproduction networks. These developments are critical as the biopharma industry prioritizes speed, quality, and global harmonization in post-pandemic capacity scaling.
Which Therapeutic Segments, Regional Clusters, and Regulatory Dynamics Are Driving Buffer Demand in Biopharma?
Monoclonal antibodies (mAbs), recombinant proteins, and advanced therapies such as CAR-T, AAV-based gene therapies, and mRNA vaccines are key drivers of buffer demand due to their high sensitivity to environmental conditions during production and storage. Each modality requires distinct buffer systems to manage formulation pH, ionic compatibility, and product stability - placing buffers at the heart of formulation development and process validation. Vaccine manufacturing and plasma-derived therapeutics are also major contributors to buffer volume growth, especially with global scale-up initiatives.North America and Western Europe remain the dominant markets, supported by strong biologics pipelines, contract manufacturing capacity, and regulatory rigor. Asia-Pacific - particularly China, India, South Korea, and Singapore - is seeing rapid growth in local biopharma manufacturing, supported by national biotech strategies and domestic production mandates. These regions are increasing buffer demand through greenfield biologics facilities, biosimilar expansion, and contract development and manufacturing organization (CDMO) partnerships. Regional sourcing, buffer localization, and GMP harmonization are becoming focal points for buffer suppliers seeking growth and risk mitigation.
Evolving regulatory expectations around raw material traceability, supplier qualification, and contamination control are further reinforcing buffer quality standards. GMP-grade certification, low bioburden thresholds, and batch-to-batch consistency are essential in securing buffer approval for regulated markets. As biopharmaceutical companies build redundancy into their supply chains, preferred vendors offering security of supply, global warehousing, and documentation compliance are gaining competitive advantage across both large-scale and niche biomanufacturing operations.
What Are the Factors Driving Growth in the Biopharma Buffer Market?
The biopharma buffer market is expanding as buffers evolve from basic reagents to strategic process enablers in the manufacture of increasingly complex, high-value biologics. Their role in safeguarding product integrity and enabling operational efficiency is becoming critical across the bioproduction value chain.Key growth drivers include the global expansion of biologics and ATMP manufacturing, increased adoption of single-use systems and continuous processing, rising demand for GMP-compliant, ready-to-use buffer systems, and growing emphasis on supply chain reliability and traceability. Innovation in formulation design and integration into digital biomanufacturing platforms is further amplifying market potential.
As biomanufacturing becomes more modular, multi-product, and digitally integrated, could buffers evolve from background inputs to frontline enablers of quality, speed, and scalability in next-generation therapeutic production?
Report Scope
The report analyzes the Biopharma Buffer market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Pre-Formulated Buffers, Customized Buffers, Concentrated Buffers, Other Types); Buffer Component (Amino Acids, Acetic Acid, Phosphate, Histidine, Other Buffer Components); Material Form (Dry, Liquid); Application (Cell Culture, Purification, Formulation); End-User (Biopharmaceutical Companies, Contract Research Organizations, Academic & Research Institutes).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Pre-Formulated Buffers segment, which is expected to reach US$2.3 Billion by 2030 with a CAGR of a 4.5%. The Customized Buffers segment is also set to grow at 7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1 Billion in 2024, and China, forecasted to grow at an impressive 8.5% CAGR to reach $1.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Biopharma Buffer Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Biopharma Buffer Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Biopharma Buffer Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aether Biomedical, Alt-Bionics, Axiles Bionics, Bionic Prosthetics & Orthotics Group, BionicM Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Biopharma Buffer market report include:
- Alfa Aesar (Thermo Fisher Scientific)
- AMRESCO LLC
- Avantor, Inc.
- BD (Becton, Dickinson and Company)
- Bioline
- Bio-Rad Laboratories, Inc.
- Biotechne/Novus Biologicals
- Corning Incorporated
- GE Healthcare
- GERBU Biotechnik GmbH
- Hamilton Company
- HiMedia Laboratories
- Lonza Group Ltd.
- Merck KGaA
- MP Biomedicals, LLC
- Promega Corporation
- Sartorius AG
- Sigma-Aldrich
- Takara Bio, Inc.
- XZL BIO-TECHNOLOGY
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alfa Aesar (Thermo Fisher Scientific)
- AMRESCO LLC
- Avantor, Inc.
- BD (Becton, Dickinson and Company)
- Bioline
- Bio-Rad Laboratories, Inc.
- Biotechne/Novus Biologicals
- Corning Incorporated
- GE Healthcare
- GERBU Biotechnik GmbH
- Hamilton Company
- HiMedia Laboratories
- Lonza Group Ltd.
- Merck KGaA
- MP Biomedicals, LLC
- Promega Corporation
- Sartorius AG
- Sigma-Aldrich
- Takara Bio, Inc.
- XZL BIO-TECHNOLOGY
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 578 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
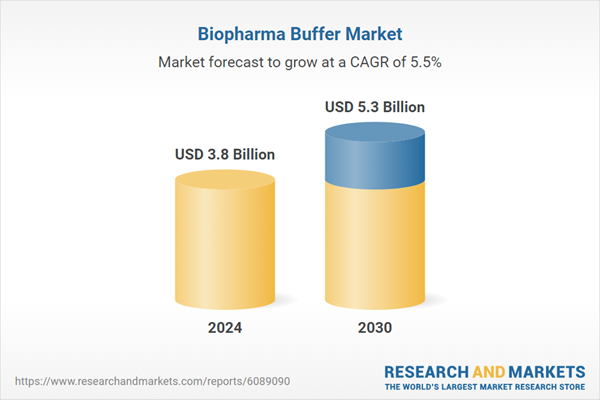
| Estimated Market Value ( USD | $ 3.8 Billion |
| Forecasted Market Value ( USD | $ 5.3 Billion |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |