Global Bacterial Vaccines Market - Key Trends & Drivers Summarized
Why Are Bacterial Vaccines Re-Emerging as Strategic Assets in Infectious Disease Control, Antimicrobial Resistance Mitigation, and Global Health Preparedness?
Bacterial vaccines are regaining prominence as global health systems confront rising antimicrobial resistance (AMR), resurgence of vaccine-preventable diseases, and ongoing vulnerability to epidemic-prone pathogens. These vaccines play a dual role in preventing morbidity and mortality while indirectly curbing antibiotic overuse by reducing the incidence of bacterial infections that often prompt empirical antimicrobial treatment. Amid growing concern over the global AMR crisis, bacterial immunization is increasingly recognized as a frontline strategy for reducing bacterial disease burden and preserving antibiotic efficacy.The continued threat posed by pathogens such as Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae type b (Hib), and Bordetella pertussis underscores the enduring importance of bacterial vaccine programs. Vaccination coverage gaps - driven by geopolitical instability, vaccine hesitancy, and health system disruptions - have led to local and regional outbreaks, prompting renewed investments in routine immunization infrastructure and booster campaigns. As bacterial infections disproportionately affect infants, the elderly, and immunocompromised populations, these vaccines are being integrated into life-course immunization frameworks by global public health authorities.
Simultaneously, emerging zoonotic and drug-resistant bacterial threats are accelerating R&D momentum and reshaping global vaccine strategies. Governments and supranational agencies are now incorporating bacterial vaccine stockpiles and targeted rollout plans into broader pandemic preparedness frameworks. This marks a shift from reactive to preventive public health postures, positioning bacterial vaccines not only as tools of disease prevention but also as essential elements of global health security infrastructure.
How Are Conjugate Technologies, Protein Subunit Platforms, and Adjuvant Innovation Advancing Vaccine Efficacy and Coverage?
Conjugate vaccine technologies have significantly improved immunogenicity against encapsulated bacteria by linking polysaccharide antigens to protein carriers, eliciting stronger and longer-lasting immune responses. Pneumococcal conjugate vaccines (PCVs), for example, have dramatically reduced invasive pneumococcal disease in children and conferred herd immunity across populations. Ongoing efforts are focused on expanding serotype coverage and optimizing schedules to enhance protection while simplifying logistics and reducing healthcare system burdens.Protein subunit vaccines are gaining traction for pathogens where polysaccharide-based approaches are less effective. These formulations, which include purified bacterial proteins or toxoids, offer targeted immune responses with strong safety profiles. Pertussis, diphtheria, and tetanus vaccines are key examples, and newer candidates are exploring conserved protein antigens to broaden strain coverage and address antigenic drift. The ability to rapidly modify subunit formulations in response to emerging variants is also strengthening their relevance in pandemic contexts.
Adjuvant innovation is further enhancing bacterial vaccine performance, particularly in older adults and immunologically challenged individuals. Novel adjuvants - such as AS01, MF59, and CpG oligodeoxynucleotides - are being incorporated to boost cellular and humoral immunity, improve dose-sparing, and generate cross-protective responses. These advances are critical for developing next-generation bacterial vaccines with durable efficacy, reduced reactogenicity, and broader population reach, especially in the context of expanding adult and booster immunization markets.
Which Target Populations, Disease Priorities, and Policy Drivers Are Shaping Demand for Bacterial Vaccination Programs?
Pediatric populations remain the core recipients of bacterial vaccines, particularly in national immunization programs that prioritize early protection against meningitis, pneumonia, sepsis, and pertussis. Widespread administration of Hib, DTP, and PCV in infancy has dramatically reduced global child mortality, especially in low- and middle-income countries (LMICs). However, disparities in access and supply chain limitations continue to influence vaccine uptake, driving efforts by Gavi and WHO to improve equity and affordability through tiered pricing and pooled procurement.Elderly adults and individuals with chronic comorbidities are an expanding target group due to their heightened susceptibility to bacterial pneumonia, bacteremia, and hospital-acquired infections. Adult immunization strategies - including pneumococcal, diphtheria-tetanus-pertussis (Tdap), and meningococcal boosters - are being adopted by high-income nations and select LMICs to reduce healthcare burden and improve quality-adjusted life years (QALYs) among aging populations. Seasonal co-administration strategies with influenza or COVID-19 vaccines are also under evaluation to streamline delivery and improve coverage.
Policy initiatives - ranging from national immunization schedule revisions to AMR mitigation plans - are driving institutional and commercial investment in bacterial vaccine scale-up. Governments are strengthening disease surveillance, catch-up campaigns, and risk-based immunization in high-transmission zones. In parallel, travel medicine, military health programs, and outbreak response protocols are incorporating bacterial vaccines as pre-emptive measures. These dynamics are reinforcing the role of bacterial vaccines as cross-cutting tools across public health, emergency preparedness, and chronic disease management domains.
How Are Global Supply Chain Investments, Multivalent Formulations, and Regulatory Innovation Accelerating Market Growth?
Strategic investments in global manufacturing and cold-chain infrastructure are enabling expanded access to bacterial vaccines, particularly in LMICs where delivery constraints have historically limited coverage. Public-private partnerships, advanced market commitments, and tech-transfer agreements are catalyzing regional vaccine production hubs to reduce reliance on centralized supply and shorten distribution timelines. These investments are also fostering greater resilience against supply shocks during health emergencies or geopolitical disruptions.The push toward multivalent formulations is streamlining immunization schedules and improving cost-efficiency. Combination vaccines targeting multiple bacterial pathogens - such as pentavalent and hexavalent formulations - are being widely adopted in pediatric programs to reduce injection burden and improve compliance. Development efforts are also underway for broad-spectrum candidates that target multiple strains within a single bacterial species, particularly for pathogens like Streptococcus pneumoniae, Salmonella typhi, and Neisseria gonorrhoeae where antigenic diversity complicates vaccine design.
Regulatory frameworks are evolving to expedite vaccine development and rollout without compromising safety or efficacy. Adaptive trial designs, real-world evidence integration, and fast-track approvals are being used to accelerate time-to-market for both pandemic-responsive and routine-use bacterial vaccines. The WHO prequalification program and regional regulatory harmonization efforts are also enhancing market entry pathways, especially for products targeting underserved populations and global health priorities.
What Role Do Pandemic Preparedness, AMR Mitigation, and Novel Target Expansion Play in Long-Term Market Strategy?
Bacterial vaccines are emerging as critical components of pandemic preparedness strategies, with a focus on pathogens with epidemic potential such as Yersinia pestis, Burkholderia pseudomallei, and Clostridioides difficile. Government funding mechanisms and biodefense programs are supporting R&D for these high-risk agents, while multilateral organizations are developing vaccine prioritization frameworks under the One Health approach - linking human, animal, and environmental health risks.As global efforts to combat AMR intensify, vaccines are being positioned as upstream interventions to reduce antibiotic reliance and slow resistance development. Vaccination prevents primary infections that would otherwise require antibiotics, indirectly reducing antimicrobial exposure and resistance pressure. This role is gaining recognition in AMR national action plans, which now include bacterial immunization coverage as a measurable objective in both clinical and community settings.
The bacterial vaccine pipeline is expanding to address unmet needs such as Helicobacter pylori, Escherichia coli (for uropathogenic and enterotoxigenic strains), Shigella, and hospital-acquired pathogens like Acinetobacter baumannii. These efforts reflect a broader strategic pivot from traditional childhood vaccines toward adult, travel, and nosocomial prevention markets. Novel delivery platforms - including intranasal, oral, and mRNA-based bacterial vaccines - are under development to enhance efficacy, accessibility, and acceptance in varied population segments.
What Are the Factors Driving Growth in the Bacterial Vaccines Market?
The bacterial vaccines market is growing as public health imperatives, antimicrobial stewardship, and global health security converge to elevate immunization as a strategic intervention against both endemic and emerging threats. Demand is expanding beyond childhood prevention into adult, travel, and nosocomial care settings.Key growth drivers include rising AMR awareness, expanded immunization guidelines, technological advancements in conjugate and protein subunit platforms, and ongoing investments in equitable access and cold-chain infrastructure. Pipeline diversification, policy alignment, and co-administration synergies with viral vaccines are reinforcing long-term momentum.
As bacterial pathogens reassert their relevance in an era of antibiotic fatigue and globalized health risks, could vaccines redefine the future of infectious disease control - not just as public health tools, but as precision instruments of systemic resilience in the post-antibiotic age?
Report Scope
The report analyzes the Bacterial Vaccines market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Vaccine Type (Pertussis / Whooping Cough Vaccines, Tetanus Vaccines, Diphtheria Vaccines, Pneumococcal Vaccines, Meningococcal Vaccines, Typhoid Vaccines, Tuberculosis Vaccines, Cholera Vaccines, Shigellosis Vaccines, Other Vaccine Types); Patient Age Group (Pediatric Vaccines, Adolescent Vaccines, Adult Vaccines, Geriatric Vaccines); Administration Route (Intramuscular, Intravenous, Subcutaneous, Other Administration Routes); Distribution Channel (Public, Private).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Pertussis / Whooping Cough Vaccines segment, which is expected to reach US$9.2 Billion by 2030 with a CAGR of a 5%. The Other Vaccine Types segment is also set to grow at 4.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $6.8 Billion in 2024, and China, forecasted to grow at an impressive 9.6% CAGR to reach $7.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Bacterial Vaccines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Bacterial Vaccines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Bacterial Vaccines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, AbbVie Inc., Allergan plc, Amgen Inc., Astellas Pharma Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 37 companies featured in this Bacterial Vaccines market report include:
- Astellas Pharma Inc.
- AstraZeneca plc
- Bavarian Nordic
- Bharat Biotech
- Biological E. Limited
- Boehringer Ingelheim GmbH
- Bristol-Myers Squibb Company
- CSL Limited (Seqirus)
- Daiichi Sankyo Company, Limited
- Emergent BioSolutions Inc.
- F. Hoffmann-La Roche Ltd.
- GlaxoSmithKline plc (GSK)
- Indian Immunologicals Ltd.
- Johnson & Johnson
- KM Biologics Co., Ltd.
- LG Chem Ltd.
- Merck & Co., Inc.
- Mitsubishi Tanabe Pharma Corporation
- Mylan N.V.
- Novartis AG
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Astellas Pharma Inc.
- AstraZeneca plc
- Bavarian Nordic
- Bharat Biotech
- Biological E. Limited
- Boehringer Ingelheim GmbH
- Bristol-Myers Squibb Company
- CSL Limited (Seqirus)
- Daiichi Sankyo Company, Limited
- Emergent BioSolutions Inc.
- F. Hoffmann-La Roche Ltd.
- GlaxoSmithKline plc (GSK)
- Indian Immunologicals Ltd.
- Johnson & Johnson
- KM Biologics Co., Ltd.
- LG Chem Ltd.
- Merck & Co., Inc.
- Mitsubishi Tanabe Pharma Corporation
- Mylan N.V.
- Novartis AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 490 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
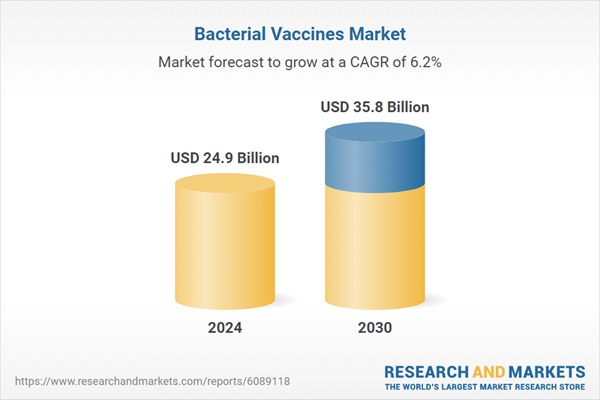
| Estimated Market Value ( USD | $ 24.9 Billion |
| Forecasted Market Value ( USD | $ 35.8 Billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |