Global Avian Influenza Vaccine Market - Key Trends & Drivers Summarized
Why Are Avian Influenza Vaccines Gaining Strategic Relevance in Biosecurity, Poultry Health Management, and Food Supply Stability?
Avian influenza vaccines are emerging as a vital line of defense in global poultry health management as highly pathogenic avian influenza (HPAI) outbreaks continue to disrupt poultry production, cross-border trade, and food security. The escalating frequency and geographic spread of HPAI strains - such as H5N1 and H5N8 - have underscored the need for vaccination strategies that go beyond culling and movement restrictions. Vaccines are being viewed not only as a disease control tool but also as a risk mitigation strategy that supports long-term continuity of supply in the global poultry value chain.The economic losses linked to avian influenza outbreaks are significant, impacting commercial poultry producers, smallholder farms, and national economies reliant on poultry exports. Mass mortality events, reduced egg production, and supply chain disruptions necessitate preemptive disease prevention approaches. Vaccination offers a cost-effective solution that helps preserve flock immunity, minimize transmission, and protect against zoonotic spillover. With growing concerns over pandemic preparedness, governments are beginning to prioritize strategic vaccine stockpiling and targeted immunization programs for high-risk zones.
Moreover, the role of avian influenza vaccines has gained renewed urgency as One Health frameworks emphasize the interconnectedness of animal health, human health, and environmental resilience. Vaccination campaigns are increasingly being integrated with surveillance programs and genetic sequencing to track viral evolution and adapt immunization strategies accordingly. As the poultry sector faces mounting pressure to ensure biosecurity, maintain animal welfare standards, and safeguard protein availability, vaccines are becoming central to the global response infrastructure.
How Are Vaccine Technologies, Strain Matching, and Delivery Platforms Advancing Efficacy and Deployment?
Vaccine development for avian influenza is evolving rapidly, with both inactivated and recombinant technologies gaining prominence. Inactivated whole-virus vaccines remain the most widely used due to their proven safety and broad immunogenicity, particularly in endemic regions. However, newer recombinant vector vaccines - utilizing platforms such as fowlpox, Newcastle disease virus (NDV), and herpesvirus of turkeys (HVT) - are enabling differentiated immune responses, DIVA (Differentiating Infected from Vaccinated Animals) compliance, and easier adaptation to emerging viral strains.Strain matching remains a critical challenge in vaccine effectiveness, as antigenic drift and reassortment frequently render existing vaccines less protective. Manufacturers are leveraging reverse genetics and genomic surveillance data to accelerate the development of strain-specific or multivalent vaccines. These approaches enable rapid customization and regulatory alignment, particularly in regions prone to variant emergence. Efforts are also underway to develop broad-spectrum or “universal” avian influenza vaccines capable of offering cross-protection against multiple subtypes.
Vaccine delivery platforms are also advancing, with injectable, spray, and in-ovo administration techniques supporting scalable immunization across different poultry species and production systems. Automated delivery systems and thermostable formulations are improving efficiency in large-scale farms, while heat-tolerant, needle-free options are being deployed in resource-limited or remote areas. The integration of vaccination with digital monitoring tools and flock-level immunization analytics is enhancing tracking and compliance in both commercial and government-led programs.
Which Poultry Segments, Production Systems, and Regional Priorities Are Driving Vaccine Demand?
Commercial layer and broiler operations are the primary users of avian influenza vaccines, given their exposure to high-density farming conditions, rapid bird turnover, and economic sensitivity to disease outbreaks. Breeder flocks and grandparent stock are also prioritized for vaccination to maintain supply chain continuity and ensure vertical protection. In contrast, backyard poultry and live bird markets, which often act as reservoirs for viral persistence, are becoming focal points for targeted government interventions and pilot vaccination programs.Free-range and organic poultry production systems - where birds have outdoor access and higher environmental exposure - are driving increased interest in preventive vaccination. These systems are at higher risk of contact with migratory birds and wild reservoirs, making proactive immunization critical for disease prevention. In regions where culling is politically or economically unviable, such as Southeast Asia, Latin America, and parts of Africa, vaccines are being deployed as a frontline control measure in both commercial and village-level operations.
Regionally, Asia-Pacific continues to lead in avian influenza vaccine consumption due to recurring HPAI outbreaks, dense poultry populations, and longstanding vaccine integration in disease control policies. China, Vietnam, Indonesia, and India have institutionalized vaccine use as part of national biosecurity frameworks. Europe is witnessing renewed adoption amid HPAI resurgence in commercial flocks and wild birds, while North America is cautiously evaluating vaccination alongside enhanced surveillance. Latin America and the Middle East are expanding their vaccination capacity to protect export-oriented poultry sectors and mitigate cross-border transmission risk.
How Are Regulatory Frameworks, Surveillance Synergies, and Trade Considerations Shaping Market Evolution?
Global regulatory agencies, including the OIE (now WOAH) and FAO, are increasingly recognizing the role of vaccines as part of holistic avian influenza management. Countries are implementing risk-based vaccination policies guided by surveillance data, regional risk mapping, and genetic sequencing. Regulatory harmonization, particularly around DIVA-compliant vaccines, is allowing countries to maintain trade transparency while deploying immunization in outbreak-prone zones.Surveillance and vaccine deployment are becoming interlinked, with integrated systems supporting real-time data collection, serological monitoring, and vaccine impact assessment. Genomic tools are enabling early detection of mutations that may impact vaccine efficacy, prompting timely reformulations. These synergies are helping authorities make informed decisions on vaccination timing, coverage, and withdrawal, minimizing both epidemiological and economic fallout from poorly matched immunization campaigns.
Trade dynamics remain a complex variable in the vaccine equation. Historically, vaccination has triggered export restrictions due to concerns over disease masking and traceability. However, this stance is shifting as science-based frameworks and DIVA strategies gain global traction. Countries are increasingly seeking trade-friendly vaccination protocols that allow for continued market access while ensuring domestic outbreak control. The evolution of trade policy will play a pivotal role in vaccine adoption strategies, particularly for export-reliant poultry economies.
What Role Do Public-Private Partnerships, Emergency Stockpiling, and R&D Pipelines Play in Strengthening Vaccine Readiness?
Public-private partnerships are proving essential in scaling production capacity, improving cold chain logistics, and advancing R&D. Governments are collaborating with animal health companies to secure vaccine stockpiles, accelerate field trials, and build surge manufacturing capability in the event of zoonotic spillover or large-scale outbreaks. These partnerships also facilitate knowledge transfer, regulatory alignment, and joint funding of platform technologies with cross-pathogen utility.Emergency preparedness is gaining momentum, with countries developing national vaccine banks and outbreak response protocols. Strategic reserves of inactivated and vector-based vaccines are being maintained for rapid deployment in containment zones. Simulation exercises and vaccine distribution drills are enhancing readiness and inter-agency coordination across agriculture, public health, and veterinary sectors. Multilateral initiatives such as the World Bank's pandemic response frameworks are further integrating avian influenza vaccines into national biosecurity roadmaps.
The R&D pipeline is diversifying, with candidates targeting improved cross-protection, thermostability, and single-dose efficacy. Research is also underway into mRNA-based avian influenza vaccines, leveraging lessons from human vaccine platforms to enhance speed and adaptability. With avian influenza remaining a high-priority zoonosis under One Health mandates, continued investment in vaccine innovation is expected to play a decisive role in global disease preparedness strategies.
What Are the Factors Driving Growth in the Avian Influenza Vaccine Market?
The avian influenza vaccine market is gaining traction as poultry sector vulnerabilities, zoonotic risk awareness, and global food security imperatives converge to reshape disease control priorities. Vaccines are no longer viewed as last-resort tools but as foundational elements of integrated, science-driven animal health management.Key growth drivers include recurring outbreaks of high-pathogenicity strains, regulatory acceptance of vaccination in trade-sensitive contexts, technological advancements in strain-matched and DIVA-compliant formulations, and expanding poultry production in high-risk regions. Public sector readiness initiatives and private sector R&D efforts are jointly reinforcing market momentum across geographies and poultry types.
As avian influenza evolves under migratory, agricultural, and climatic pressures, could vaccine-enabled resilience redefine how the world safeguards poultry supply chains and prepares for the next zoonotic frontier?
Report Scope
The report analyzes the Avian Influenza Vaccine market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Inactivated, Live Attenuated, Recombinant, Vector-based, Other Types); Application (Chicken, Duck, Goose, Turkey, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Inactivated Vaccine segment, which is expected to reach US$800.9 Million by 2030 with a CAGR of a 7.4%. The Live Attenuated Vaccine segment is also set to grow at 5.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $352.5 Million in 2024, and China, forecasted to grow at an impressive 10.8% CAGR to reach $402.1 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Avian Influenza Vaccine Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Avian Influenza Vaccine Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Avian Influenza Vaccine Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ABB Ltd., AgileX Robotics, Boston Dynamics, Clearpath Robotics, Doosan Robotics and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 37 companies featured in this Avian Influenza Vaccine market report include:
- AstraZeneca
- Bayer AG
- Bimeda Inc.
- Boehringer Ingelheim GmbH
- Ceva Santé Animale
- CSL Seqirus
- Elanco Animal Health
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- GlaxoSmithKline plc (GSK)
- HIPRA
- ID Biomedical Corporation
- Johnson & Johnson
- Mallinckrodt Pharmaceuticals
- Medion
- Merck & Co., Inc.
- Microgen
- Moderna, Inc.
- Novartis AG
- Novavax, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AstraZeneca
- Bayer AG
- Bimeda Inc.
- Boehringer Ingelheim GmbH
- Ceva Santé Animale
- CSL Seqirus
- Elanco Animal Health
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- GlaxoSmithKline plc (GSK)
- HIPRA
- ID Biomedical Corporation
- Johnson & Johnson
- Mallinckrodt Pharmaceuticals
- Medion
- Merck & Co., Inc.
- Microgen
- Moderna, Inc.
- Novartis AG
- Novavax, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 286 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
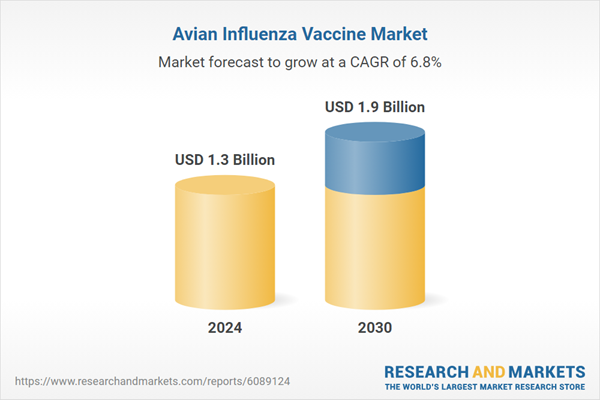
| Estimated Market Value ( USD | $ 1.3 Billion |
| Forecasted Market Value ( USD | $ 1.9 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |