Global Artificial Intelligence / Machine Learning in Medical Devices Market - Key Trends & Drivers Summarized
Why Are AI and Machine Learning Transforming the Performance, Utility, and Design of Medical Devices?
Artificial Intelligence (AI) and Machine Learning (ML) are redefining the capabilities of medical devices, enabling real-time data analysis, predictive diagnostics, and adaptive therapeutic interventions across multiple clinical disciplines. By embedding intelligent algorithms into hardware platforms, medical devices are evolving from passive tools into active decision-support and automation systems. AI/ML integration is now central to digital health transformation, offering enhanced clinical accuracy, workflow efficiency, and personalized care delivery.Diagnostic devices leveraging ML can rapidly analyze imaging, waveform, or sensor data to detect anomalies, prioritize critical cases, and reduce diagnostic variability. In radiology, for instance, AI-augmented imaging systems detect early-stage tumors, classify fractures, and segment anatomical structures with precision that rivals or augments human interpretation. Meanwhile, ML-driven wearable and implantable devices continuously monitor physiological parameters - such as ECG, glucose levels, or oxygen saturation - to predict deterioration, flag outliers, and enable preemptive care interventions.
In therapeutic applications, AI algorithms support device-guided surgery, dosage optimization, and closed-loop stimulation systems. Robotic-assisted surgical platforms use ML to refine motion control and map anatomical nuances in real-time. AI-powered infusion pumps and neuromodulation devices adjust treatment regimens dynamically based on patient-specific feedback loops. As regulatory bodies approve a growing number of AI/ML-enabled software as a medical device (SaMD) solutions, intelligent device functionality is becoming a clinical imperative rather than a competitive novelty.
How Are Algorithm Validation, Data Ecosystems, and Edge AI Enhancing Adoption of AI/ML in Medical Devices?
Algorithm validation and model transparency are foundational to trust and adoption in medical AI. Developers are investing in large-scale, multi-institutional training datasets and real-world evidence (RWE) to ensure algorithm generalizability across patient demographics and clinical settings. Techniques such as federated learning and explainable AI (XAI) are gaining traction, enabling continuous model improvement without compromising data privacy or interpretability. Regulatory agencies, including the FDA, are establishing adaptive frameworks to approve ML algorithms that evolve post-deployment - supporting safer, lifecycle-managed innovation.Data integration is further accelerating AI/ML deployment across the device landscape. Medical devices now interface with cloud platforms, hospital information systems (HIS), electronic health records (EHR), and wearable ecosystems to contextualize sensor data and enhance clinical relevance. Interoperability standards such as HL7 FHIR are facilitating cross-platform data exchange, allowing AI algorithms to draw insights from multimodal sources. These insights not only improve diagnostic and therapeutic precision but also contribute to longitudinal patient management.
Edge AI - the deployment of machine learning models directly on-device - is a critical enabler for latency-sensitive applications such as point-of-care diagnostics, portable ultrasound, or remote monitoring wearables. By minimizing reliance on continuous cloud connectivity, edge AI ensures responsiveness, data privacy, and operability in low-bandwidth or decentralized environments. Miniaturization of processors, advances in low-power computing, and embedded ML chipsets are supporting the growth of intelligent, self-contained medical devices capable of real-time autonomous decision-making.
Which Clinical Domains and Regional Markets Are Driving AI/ML Adoption in Medical Devices?
Radiology, cardiology, neurology, and oncology represent the most advanced domains in AI/ML-driven medical device adoption, owing to their high imaging intensity, data complexity, and need for diagnostic accuracy. AI-enhanced ultrasound, MRI, CT, and PET systems are being deployed in hospitals and imaging centers to automate workflows, reduce scan-to-diagnosis time, and support overburdened radiology departments. In cardiology, AI-enabled ECG monitors, wearable arrhythmia detectors, and heart failure prediction systems are improving chronic disease surveillance and risk stratification.Surgical robotics, anesthesia monitoring, and critical care systems are incorporating ML algorithms to optimize intraoperative decisions, ventilator management, and post-operative recovery pathways. In consumer health and home care, AI-enabled devices such as smart glucometers, digital stethoscopes, and fall detection wearables are bridging gaps in continuity of care and enabling scalable chronic disease management. Dental and ophthalmic devices are also integrating AI for image analysis, procedural planning, and risk detection, expanding application boundaries.
North America dominates the AI/ML-enabled medical device landscape, driven by early regulatory approvals, strong digital health infrastructure, and a robust base of AI startups and MedTech incumbents. Europe follows closely, with emphasis on ethical AI, data privacy compliance (GDPR), and public-private innovation frameworks. The Asia-Pacific region is witnessing rapid uptake, particularly in China, Japan, South Korea, and India - where government investments, digital transformation agendas, and large untapped patient datasets are accelerating AI/ML integration. Growth in Latin America, the Middle East, and Africa is expected to be driven by mobile diagnostics, telehealth-linked devices, and public health infrastructure modernization.
How Are Regulatory Standards, Business Models, and Clinical Integration Strategies Reshaping the Competitive Landscape?
Global regulatory frameworks are evolving to accommodate the unique lifecycle of AI/ML-enabled devices. Regulatory bodies are issuing guidelines for algorithm transparency, real-world validation, and change management to address continuous learning models. The FDA's action plan for AI/ML medical software and the EU MDR's classification criteria are helping define pathways for safe, repeatable deployment. These frameworks are encouraging early-stage AI developers to incorporate regulatory strategy at the core of product design.Business models are shifting toward AI-as-a-service, subscription-based analytics, and outcomes-based contracting. Device manufacturers are embedding AI modules as differentiated add-ons - offering hospitals predictive insights, workflow efficiency, and diagnostics-as-a-platform capabilities. Strategic alliances with cloud providers, EHR vendors, and clinical AI startups are enabling MedTech firms to scale AI/ML integration without rebuilding full-stack capabilities in-house. Reimbursement strategies are emerging for AI-driven diagnostics and triage tools, further supporting commercialization viability.
Successful adoption hinges on seamless clinical integration. Developers are focusing on intuitive user interfaces, clinician-in-the-loop controls, and integration with existing workflows to avoid alert fatigue or workflow disruption. Training programs, real-world case studies, and post-market surveillance systems are critical in building clinician trust and ensuring safe adoption. As competitive intensity rises, AI-enabled device differentiation is increasingly dependent on clinical relevance, interoperability, and regulatory agility rather than algorithm sophistication alone.
What Are the Factors Driving Growth in the AI/ML in Medical Devices Market?
The AI/ML in medical devices market is growing rapidly, driven by rising healthcare digitization, growing volumes of patient data, and clinical demand for precision, automation, and decision support. The convergence of computing power, data availability, and algorithm maturity is enabling medical devices to become intelligent, context-aware tools that proactively assist clinicians across diagnosis, monitoring, and treatment.Advances in edge AI, explainable algorithms, and regulatory frameworks are making AI-enabled devices safer, more scalable, and more commercially viable. As clinical workflows demand actionable insights at the point of care, and as payers push for value-based outcomes, intelligent medical devices are aligning with broader healthcare system transformation goals.
Looking ahead, the market's trajectory will depend on how effectively AI-enabled devices integrate into clinical decision-making, balance innovation with safety, and demonstrate measurable improvements in patient outcomes. As medical devices evolve from passive instruments to cognitive collaborators, could AI/ML define the next frontier of adaptive, personalized, and data-driven healthcare delivery?
Report Scope
The report analyzes the Artificial Intelligence / Machine Learning in Medical Devices market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (System / Hardware, Software-As-A Medical Device); Clinical Area (Radiology, Cardiology, Hematology, Other Clinical Areas).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the System / Hardware segment, which is expected to reach US$8.8 Billion by 2030 with a CAGR of a 18.2%. The Software-As-A Medical Device segment is also set to grow at 22.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.2 Billion in 2024, and China, forecasted to grow at an impressive 18.8% CAGR to reach $2.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Artificial Intelligence / Machine Learning in Medical Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Artificial Intelligence / Machine Learning in Medical Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Artificial Intelligence / Machine Learning in Medical Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AlloSource, Baxter International Inc., Biogennix, Biomatlante, Bone Support AB and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Artificial Intelligence / Machine Learning in Medical Devices market report include:
- AEYE Health
- AliveCor
- Atomwise
- Behold.ai
- BenevolentAI
- BioSerenity
- Butterfly Network
- Clarius Mobile Health
- CorticoMetrics
- Cyclica
- Eko Health
- Empatica
- Enlitic
- Exscientia
- GE Healthcare
- HeartFlow
- iCAD Inc.
- Lantheus Holdings
- Medtronic
- NVIDIA
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AEYE Health
- AliveCor
- Atomwise
- Behold.ai
- BenevolentAI
- BioSerenity
- Butterfly Network
- Clarius Mobile Health
- CorticoMetrics
- Cyclica
- Eko Health
- Empatica
- Enlitic
- Exscientia
- GE Healthcare
- HeartFlow
- iCAD Inc.
- Lantheus Holdings
- Medtronic
- NVIDIA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 134 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
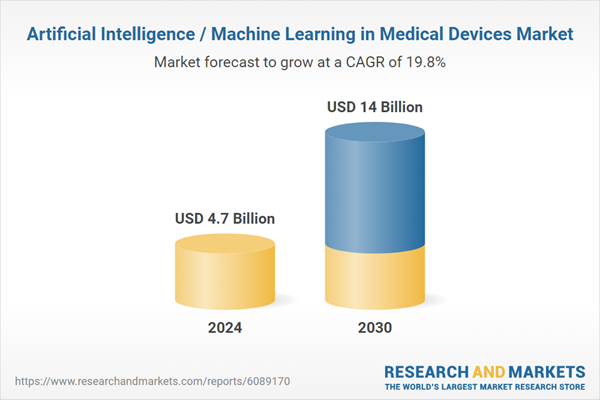
| Estimated Market Value ( USD | $ 4.7 Billion |
| Forecasted Market Value ( USD | $ 14 Billion |
| Compound Annual Growth Rate | 19.8% |
| Regions Covered | Global |