Global Aortic Endografts Market - Key Trends & Drivers Summarized
Why Are Aortic Endografts Redefining the Standard of Care in Aneurysm Repair and Vascular Disease Management?
Aortic endografts - used in endovascular aneurysm repair (EVAR) and thoracic endovascular aortic repair (TEVAR) - have revolutionized the treatment of aortic aneurysms by enabling minimally invasive interventions that significantly reduce perioperative risk, hospital stay, and recovery time. These graft-stent hybrids are deployed inside the aorta to exclude aneurysmal sacs from blood flow, preventing rupture while preserving vascular continuity. Their use has become central in managing abdominal aortic aneurysms (AAA) and thoracic aortic aneurysms (TAA), especially in high-risk or elderly patients who are not ideal candidates for open surgical repair.The transition from open surgery to EVAR and TEVAR is supported by strong clinical outcomes, including lower procedural mortality, reduced postoperative complications, and faster patient mobilization. Aortic endografts are increasingly being used in both elective and emergency settings, including ruptured aneurysms and traumatic aortic injuries, underscoring their growing relevance in acute vascular care. Their precision-engineered architecture - comprising self-expanding metallic stents and biocompatible graft fabrics - enables tailored solutions for complex anatomies and challenging vascular access pathways.
Beyond aneurysm exclusion, aortic endografts are gaining traction in hybrid and branched endovascular procedures for aortic dissections, penetrating atherosclerotic ulcers, and other pathologies involving the thoracic and abdominal aorta. These expanded indications, along with supportive clinical data and evolving surgical expertise, are driving greater adoption among vascular surgeons and interventional radiologists. As patient selection broadens and device versatility improves, aortic endografts are redefining therapeutic possibilities in vascular medicine.
How Are Device Design Innovations and Imaging Integration Enhancing Procedural Precision and Outcomes?
Advancements in device design are transforming aortic endografts into highly customizable, anatomy-conforming implants that accommodate wide variations in aortic diameter, tortuosity, and branch vessel proximity. Modular graft systems, low-profile delivery sheaths, and repositionable stent components are improving navigation through narrow or calcified iliac arteries, enabling safe deployment even in patients with hostile anatomies. The integration of suprarenal fixation, active sealing rings, and fenestrated or branched designs is extending applicability to juxtarenal and thoracoabdominal aneurysms previously considered unsuitable for EVAR or TEVAR.The role of imaging has become integral to the success of aortic endograft placement. High-resolution preoperative CT angiography, 3D vascular mapping, and intraoperative fluoroscopy or cone-beam CT enable precise device sizing, landing zone identification, and real-time graft navigation. Advanced fusion imaging platforms now offer dynamic guidance by overlaying pre-op scans onto live angiographic views, significantly reducing contrast use, radiation exposure, and operative time. These tools are helping operators achieve optimal sealing and alignment, while minimizing endoleak risk and procedural complications.
Material innovation is further enhancing graft durability and biocompatibility. Improvements in graft fabrics, such as woven polyester and ePTFE with heparin-bonded surfaces, are improving thromboresistance, minimizing inflammatory response, and extending implant longevity. Long-term performance is being closely linked to material fatigue resistance, radial strength, and conformability, especially in younger or more active patients. As device technology advances, the focus is shifting toward reducing reintervention rates and improving long-term clinical durability of endograft therapy.
Which Clinical Indications and Regional Markets Are Driving Growth in Aortic Endograft Adoption?
The most common indication for aortic endograft use remains abdominal aortic aneurysm (AAA) repair, where EVAR has become the standard of care in many high-income markets. Thoracic aortic aneurysm (TAA) repair using TEVAR is also gaining adoption, especially for descending aorta involvement and aortic arch extension cases requiring complex branched grafts. Additional clinical indications include traumatic aortic transections, chronic type B dissections, and hybrid repairs in multilevel aortic disease - broadening procedural volumes and device utilization across vascular specialties.Demand is particularly strong in countries with aging populations, high cardiovascular disease prevalence, and advanced hospital infrastructure. North America and Western Europe are mature markets with high procedural penetration, sustained by favorable reimbursement policies, robust screening programs, and specialist surgical training. Meanwhile, Asia-Pacific is witnessing accelerated growth due to rising awareness, expanding vascular programs, and infrastructure upgrades in China, India, South Korea, and Japan. Latin America and Eastern Europe are also increasing adoption through government-funded cardiac and vascular care expansion initiatives.
Public health policies that emphasize early aneurysm screening, especially among men aged 65 and older, are reinforcing demand in developed countries. In parallel, private healthcare networks and specialty hospitals in emerging markets are investing in endovascular capabilities to attract high-value cardiovascular procedures. As health systems prioritize minimally invasive care, short inpatient stays, and outcome-based reimbursement, aortic endografts are increasingly positioned as the preferred option in aneurysm repair and vascular trauma response.
How Are Regulatory Trends, Competitive Differentiation, and Post-Market Surveillance Influencing the Market Landscape?
Regulatory authorities are refining approval pathways and surveillance protocols for aortic endografts to ensure both procedural safety and long-term device performance. In major markets, regulatory frameworks now require robust evidence of durability, anatomical compatibility, and procedural reproducibility. Post-market surveillance programs - including device registries, mandatory follow-up imaging, and adverse event reporting - are shaping product lifecycle management and manufacturer accountability.Manufacturers are differentiating through innovations in graft design, delivery system ergonomics, and endoleak mitigation strategies. Competitive positioning is being driven by ease of deployment, anatomical versatility, and device compatibility with hybrid and branched configurations. Some players are offering physician-modifiable or off-the-shelf branched endografts to reduce manufacturing lead times and improve emergency use readiness. Strategic partnerships with imaging companies and training institutions are enhancing brand loyalty and procedural standardization.
With greater focus on outcomes and cost-effectiveness, value-based procurement models are emerging in several healthcare systems. Hospitals and payers are evaluating aortic endografts not only by their unit cost but by total treatment cost, including reintervention rates, long-term durability, and imaging burden. As healthcare delivery moves toward precision medicine and real-time procedural support, devices that integrate smart feedback, imaging compatibility, and adaptive deployment will command a premium in the competitive landscape.
What Are the Factors Driving Growth in the Aortic Endografts Market?
The aortic endografts market is expanding steadily, driven by a confluence of clinical demand, technological advancement, and health system preferences for minimally invasive therapies. The growing burden of aortic aneurysms, improvements in vascular diagnostics, and continued migration from open surgery to EVAR and TEVAR procedures are core market drivers. Key growth enablers include the rising incidence of vascular trauma, aging demographics, and expanded indications for endovascular repair in complex and urgent cases.Ongoing device innovation, integration with advanced imaging, and enhanced physician training are accelerating procedural adoption across both developed and emerging healthcare systems. Strategic investments in hybrid ORs, vascular centers of excellence, and multidisciplinary care models are reinforcing institutional readiness for endograft-based interventions. Market expansion is further supported by streamlined regulatory approvals and targeted reimbursement policies that recognize the clinical and economic value of minimally invasive vascular repair.
Looking ahead, the trajectory of the aortic endografts market will be shaped by how effectively device platforms address anatomical complexity, long-term durability, and procedure-specific customization. As endovascular care continues to evolve, could aortic endografts emerge as the definitive cornerstone of 21st-century aortic disease management?
Report Scope
The report analyzes the Aortic Endografts market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Abdominal Aortic Grafts, Thoracic Aortic Grafts); Material (Metallic Endografts, Polymeric Endografts); End-Use (Hospitals, Ambulatory Surgery Centers, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Abdominal Aortic Grafts segment, which is expected to reach US$2.4 Billion by 2030 with a CAGR of a 3.1%. The Thoracic Aortic Grafts segment is also set to grow at 5.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $796.1 Million in 2024, and China, forecasted to grow at an impressive 6.8% CAGR to reach $728.5 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Aortic Endografts Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Aortic Endografts Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Aortic Endografts Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ACL Staticide, Inc., Aroindia Electromech Pvt. Ltd., Bentwood (GH Grinding & Brewing Solutions), Botron Company Inc., Changzhou Titan Decoration Materials Co., Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Aortic Endografts market report include:
- Abbott Laboratories
- Altura Medical Inc.
- Aptus Endosystems Inc.
- Artivion Inc.
- B. Braun Melsungen AG
- Bentley InnoMed GmbH
- Biomerics LLC
- Boston Scientific Corporation
- Braile Biomédica
- Cardiatis S.A.
- Cardinal Health Inc.
- Cook Medical Inc.
- Cordis Corporation
- CryoLife Inc.
- Edwards Lifesciences
- Endologix LLC
- Endospan Ltd.
- Getinge AB
- JOTEC GmbH
- Koninklijke Philips N.V.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Altura Medical Inc.
- Aptus Endosystems Inc.
- Artivion Inc.
- B. Braun Melsungen AG
- Bentley InnoMed GmbH
- Biomerics LLC
- Boston Scientific Corporation
- Braile Biomédica
- Cardiatis S.A.
- Cardinal Health Inc.
- Cook Medical Inc.
- Cordis Corporation
- CryoLife Inc.
- Edwards Lifesciences
- Endologix LLC
- Endospan Ltd.
- Getinge AB
- JOTEC GmbH
- Koninklijke Philips N.V.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 368 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
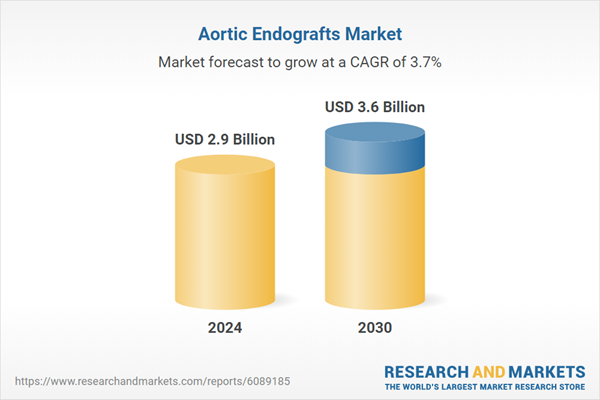
| Estimated Market Value ( USD | $ 2.9 Billion |
| Forecasted Market Value ( USD | $ 3.6 Billion |
| Compound Annual Growth Rate | 3.7% |
| Regions Covered | Global |