Global Antimicrobial Therapeutics Market - Key Trends & Drivers Summarized
Why Are Antimicrobial Therapeutics Central to Global Health, Infection Control, and Clinical Outcomes?
Antimicrobial therapeutics are foundational to modern medicine, enabling the treatment and prevention of infections caused by bacteria, viruses, fungi, and parasites. Their role extends far beyond treating acute illnesses - they are essential for safeguarding outcomes in routine surgeries, immunosuppressive therapies, organ transplants, and cancer care. By targeting and neutralizing pathogenic microorganisms, these drugs reduce morbidity, mortality, and transmission rates, forming the backbone of public health defense systems globally.Antibiotics remain the most widely used class within this group, addressing bacterial infections ranging from community-acquired pneumonia to sepsis and multidrug-resistant tuberculosis. Antivirals play a pivotal role in controlling infections such as HIV, hepatitis, and influenza, while antifungals and antiparasitics are indispensable in oncology and tropical disease management. In hospital settings, antimicrobial therapeutics underpin infection control protocols, especially in intensive care units, transplant centers, and oncology wards, where immunocompromised patients are at heightened risk.
Beyond clinical care, antimicrobial agents are vital in agricultural biosecurity, veterinary medicine, and biodefense preparedness. Their broad utility has made them among the most widely prescribed therapeutic categories globally. However, rising antimicrobial resistance (AMR), emerging infectious disease threats, and global pandemics are exposing the vulnerabilities of current therapeutic arsenals, making antimicrobial innovation and stewardship increasingly urgent on the global health agenda.
How Are Innovation Strategies and Scientific Advances Shaping the Future of Antimicrobial Development?
The discovery and development of new antimicrobial therapeutics have long been constrained by biological complexity, regulatory hurdles, and low commercial incentives. However, recent advances in genomics, high-throughput screening, structure-based drug design, and microbiome research are reinvigorating drug discovery pipelines. Novel mechanisms of action - such as inhibition of bacterial virulence factors, quorum sensing blockers, and host-pathogen interface disruptors - are being explored to circumvent resistance development and prolong therapeutic efficacy.Long-acting injectables, inhaled antimicrobials, and nanoparticle-based delivery systems are improving pharmacokinetics, targeting, and bioavailability - particularly in hard-to-reach infections like osteomyelitis, endocarditis, and chronic lung disease. Additionally, antibody-based antimicrobials and bacteriophage therapies are gaining attention as precision alternatives to broad-spectrum antibiotics. These biologics offer the potential to target specific pathogens while preserving the host microbiota, reducing the risk of resistance and collateral damage.
Innovation is also being driven by collaborations between public agencies, global health NGOs, and private developers under new economic models such as push incentives (grants, R&D subsidies) and pull mechanisms (market entry rewards, subscription payments). Programs like CARB-X, GARDP, and the AMR Action Fund are funding early-stage research and derisking development pipelines for novel antimicrobials targeting critical pathogens. As new paradigms emerge for sustainable innovation in this high-need, low-return domain, the market is gradually repositioning itself toward addressing both scientific and commercial viability gaps.
Which Therapeutic Areas and Regional Markets Are Driving Demand for Antimicrobial Solutions?
Respiratory infections, urinary tract infections (UTIs), skin and soft tissue infections, and hospital-acquired infections (HAIs) remain the largest therapeutic areas for antimicrobial use. In the post-pandemic landscape, viral respiratory diseases - including influenza, COVID-19, and RSV - have heightened awareness of the need for broad-spectrum antiviral preparedness. The continued prevalence of bacterial pneumonia, tuberculosis, gonorrhea, and multidrug-resistant pathogens in both inpatient and outpatient settings is sustaining strong baseline demand across all antimicrobial classes.High-burden disease regions - particularly in Asia-Pacific, Sub-Saharan Africa, and Latin America - are seeing rising usage due to expanding access to essential medicines, growing antimicrobial stewardship programs, and government-led infection control initiatives. In developed economies, rising rates of AMR, aging populations, and increasing hospital procedural volumes are driving demand for next-generation therapeutics that address multidrug-resistant organisms such as MRSA, CRE, and VRE. Concurrently, community-level viral outbreaks and chronic disease comorbidities are amplifying the need for sustained, broad-based antimicrobial availability.
Pediatric and geriatric populations represent critical demand segments, given their higher susceptibility to infections and frequent exposure to polypharmacy. Oncology, critical care, and transplant patients also generate high-volume usage due to immunocompromised conditions that necessitate prophylactic and empiric antimicrobial use. With the global healthcare focus shifting toward universal health coverage and pandemic resilience, antimicrobial therapeutics remain at the center of both essential and emergency care frameworks worldwide.
How Are Resistance Pressures, Regulatory Frameworks, and Stewardship Efforts Reshaping Market Dynamics?
Antimicrobial resistance is reshaping the market by accelerating the obsolescence of existing drug classes and driving up healthcare costs, morbidity, and mortality rates. AMR now claims an estimated 1.2 million lives annually and is projected to surpass cancer as a leading cause of death by 2050 if unchecked. Regulatory bodies are mandating stronger stewardship policies, surveillance programs, and prescription controls to slow resistance development and promote rational drug use. These shifts are directly influencing prescribing behavior and procurement priorities across healthcare systems.Agencies such as the FDA, EMA, and WHO are implementing streamlined regulatory pathways - including Qualified Infectious Disease Product (QIDP) designations and accelerated review mechanisms - to fast-track urgently needed antimicrobials. At the same time, policies such as antibiotic use benchmarking, formulary restrictions, and hospital-level antibiogram tracking are encouraging precision usage and reducing empirical overuse. Pharmaceutical developers are under growing pressure to demonstrate not only efficacy but also ecological safety and low resistance potential in new product launches.
Stewardship and surveillance are becoming integral to hospital operations, often linked to reimbursement and accreditation standards. Digital tools and diagnostic innovations - such as rapid pathogen identification, susceptibility testing, and electronic prescribing audits - are being integrated into stewardship programs to support evidence-based decision-making. These interventions, combined with educational efforts and national action plans, are redefining the value proposition of antimicrobials from commodity drugs to strategically managed therapeutic assets.
What Are the Factors Driving Growth in the Antimicrobial Therapeutics Market?
The global antimicrobial therapeutics market is expanding, driven by the growing prevalence of infectious diseases, rising antimicrobial resistance, and renewed public health investment post-COVID-19. Continued demand in primary care, hospital settings, and specialty medicine underscores the essential role of antimicrobials in both acute intervention and chronic care support. Growth is further supported by emerging infectious disease threats, aging populations, global travel, and expanded access to care in developing markets.While resistance, pricing pressure, and R&D risk continue to challenge commercial sustainability, the landscape is being reshaped by collaborative funding models, innovative pipeline strategies, and evolving regulatory support. The rise of targeted biologics, novel delivery formats, and digital diagnostics is redefining what constitutes effective, next-generation antimicrobial care. Simultaneously, public awareness, policy mandates, and ESG-aligned pharma strategies are reinforcing the need for sustainable antibiotic development and responsible market deployment.
Looking ahead, the future of the antimicrobial therapeutics market will depend on how successfully the sector can balance innovation, access, and resistance containment. As pathogens evolve and treatment gaps widen, could a new generation of precision-designed, stewardship-integrated antimicrobials emerge as the frontline defenders of global health security?
Report Scope
The report analyzes the Antimicrobial Therapeutics market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Drug Class (Antibacterials, Antivirals, Antifungals, Antiparasitics); Disease Indication (Respiratory Infections, Urinary Tract Infections, Skin & Soft Tissue infections, Sexually Transmitted Infections, Gastrointestinal Infections, Central Nervous System Infections, Other Disease Indications); Administration Route (Oral, Topical, Injectable, Other Administration Routes); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Antibacterials segment, which is expected to reach US$58.4 Billion by 2030 with a CAGR of a 5.5%. The Antivirals segment is also set to grow at 5.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $30.8 Billion in 2024, and China, forecasted to grow at an impressive 8.7% CAGR to reach $31.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Antimicrobial Therapeutics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Antimicrobial Therapeutics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Antimicrobial Therapeutics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Ajanta Pharma Limited, Almirall S.A., AstraZeneca plc, Bayer AG, Boehringer Ingelheim GmbH and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Antimicrobial Therapeutics market report include:
- AbbVie Inc.
- Allecra Therapeutics GmbH
- Aridis Pharmaceuticals, Inc.
- Astellas Pharma Inc.
- AstraZeneca plc
- Basilea Pharmaceutica Ltd.
- Bayer AG
- BioVersys AG
- Bristol-Myers Squibb Company
- Cadila Pharmaceuticals Ltd.
- Cipla Ltd.
- CSL Limited
- Eli Lilly and Company
- Emergent BioSolutions Inc.
- F. Hoffmann-La Roche Ltd
- Gilead Sciences, Inc.
- GlaxoSmithKline plc
- Inovio Pharmaceuticals, Inc.
- Johnson & Johnson
- KYORIN Pharmaceutical Co., Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Allecra Therapeutics GmbH
- Aridis Pharmaceuticals, Inc.
- Astellas Pharma Inc.
- AstraZeneca plc
- Basilea Pharmaceutica Ltd.
- Bayer AG
- BioVersys AG
- Bristol-Myers Squibb Company
- Cadila Pharmaceuticals Ltd.
- Cipla Ltd.
- CSL Limited
- Eli Lilly and Company
- Emergent BioSolutions Inc.
- F. Hoffmann-La Roche Ltd
- Gilead Sciences, Inc.
- GlaxoSmithKline plc
- Inovio Pharmaceuticals, Inc.
- Johnson & Johnson
- KYORIN Pharmaceutical Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 494 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
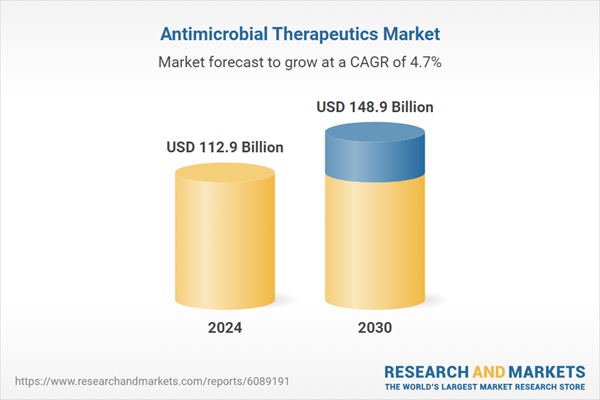
| Estimated Market Value ( USD | $ 112.9 Billion |
| Forecasted Market Value ( USD | $ 148.9 Billion |
| Compound Annual Growth Rate | 4.7% |
| Regions Covered | Global |