Global Acute Spinal Cord Injury (SCI) Treatment Market - Key Trends & Drivers Summarized
Why Is Acute Spinal Cord Injury a Critical Therapeutic Priority in Emergency, Trauma, and Neurorehabilitation Medicine?
Acute spinal cord injury (SCI) is a sudden, traumatic event resulting in partial or complete disruption of sensory, motor, and autonomic functions below the injury site. It represents one of the most complex and devastating neurological emergencies, often arising from vehicular accidents, falls, sports injuries, or violence. Immediate intervention is vital, as irreversible secondary damage - driven by ischemia, inflammation, and excitotoxicity - can rapidly follow the initial trauma, exacerbating long-term disability.With high societal and economic burden, acute SCI is increasingly prioritized in trauma and neurosurgical protocols worldwide. Early recognition, surgical stabilization, and evidence-based pharmacologic and rehabilitative strategies are essential to maximizing functional outcomes, reducing complications, and improving quality of life for affected individuals.
How Are Innovations in Surgical Intervention, Pharmacotherapy, and Neuroprotection Advancing SCI Care?
Surgical decompression and stabilization remain cornerstone treatments, with technological advances enabling more precise and minimally invasive approaches that reduce operative risk and support early mobilization. Spinal navigation, intraoperative imaging, and neuro-monitoring are improving surgical outcomes by enhancing alignment accuracy and minimizing iatrogenic damage.Pharmacological approaches, including high-dose methylprednisolone in select cases, are being reassessed, while emerging neuroprotective therapies are targeting inflammation, apoptosis, and oxidative stress. Research is advancing into agents such as riluzole, minocycline, and stem cell-derived biologics to limit secondary damage and promote neuroregeneration. In parallel, intensive rehabilitation and assistive technologies such as exoskeletons and electrical stimulation devices are enhancing neuroplasticity and functional recovery.
Which Patient Demographics and Healthcare Systems Are Driving Demand for Acute SCI Treatment?
Young adult males account for a large proportion of acute SCI cases globally due to their higher exposure to risk-prone activities. However, aging populations are contributing to a rise in SCI from low-impact falls, especially in developed countries. Emergency departments, trauma centers, and specialized neurorehabilitation units are the primary points of care, with long-term management often requiring multidisciplinary follow-up and community-based support.North America leads in terms of incidence tracking, advanced trauma response systems, and availability of post-acute care infrastructure. Europe follows with robust neurorehabilitation programs and ongoing clinical trials in SCI therapeutics. Asia-Pacific is witnessing rising caseloads amid rapid urbanization and increasing motor vehicle accidents, driving regional investments in trauma care and spinal surgery capabilities. In Latin America and parts of Africa, gaps in early intervention and long-term support remain critical challenges.
How Are Health Policies, Research Funding, and Assistive Technologies Influencing Market Development?
Policy-level recognition of SCI as a public health concern is accelerating investment in emergency medical services, surgical capacity, and long-term rehabilitation. National trauma registries, clinical guidelines, and funding for SCI centers of excellence are facilitating standardized care delivery and research participation. Insurance coverage for surgical intervention, rehabilitation, and mobility aids is also improving in several regions, expanding treatment access.Significant funding is being directed toward SCI research, particularly in neuroregeneration, spinal cord implants, and advanced rehabilitation robotics. Collaborative efforts between academia, biotech firms, and government agencies are pushing the boundaries of clinical innovation. Wearable assistive devices, brain-computer interfaces, and neuroprosthetics are offering new dimensions of independence and recovery for individuals with SCI, creating future opportunities for integrated treatment pathways.
What Are the Factors Driving Growth in the Acute SCI Treatment Market?
The acute SCI treatment market is expanding due to increasing global trauma incidence, heightened awareness, improved emergency response systems, and rapid advancements in surgical and neuroprotective strategies. Growth is also supported by the rising availability of specialized neurorehabilitation services and innovation in assistive technologies that support functional restoration. As clinical guidelines evolve and research breakthroughs accelerate, the standard of care for acute SCI is undergoing meaningful transformation.Looking ahead, the market's trajectory will be shaped by how effectively emerging therapies, surgical innovations, and long-term care models converge to improve outcomes and restore autonomy. As global focus intensifies on spinal cord regeneration and functional recovery, could acute SCI treatment become a cornerstone of next-generation neurotrauma and rehabilitation medicine?
Report Scope
The report analyzes the Acute Spinal Cord Injury market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Complete Spinal Cord Injury, Incomplete Spinal Cord Injury); End-Use (Hospitals, Trauma Centers).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Complete Spinal Cord Injury segment, which is expected to reach US$6.2 Billion by 2030 with a CAGR of a 5.2%. The Incomplete Spinal Cord Injury segment is also set to grow at 2.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.8 Billion in 2024, and China, forecasted to grow at an impressive 8.3% CAGR to reach $1.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Acute Spinal Cord Injury Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Acute Spinal Cord Injury Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Acute Spinal Cord Injury Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie Inc., Amanta Healthcare, Amgen Inc., AM-Pharma B.V., Angion Biomedica Corp. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Acute Spinal Cord Injury market report include:
- AbbVie Inc.
- Acorda Therapeutics, Inc.
- Asterias Biotherapeutics, Inc.
- Athersys, Inc.
- Axoltis Pharma
- BioArctic AB
- BioAxone BioSciences, Inc.
- BioTime, Inc.
- Geron Corporation
- Histocell S.L.
- InVivo Therapeutics Holdings Corp
- Kringle Pharma, Inc.
- Lineage Cell Therapeutics, Inc.
- Merz Therapeutics
- Mitsubishi Tanabe Pharma Corp.
- Neuralstem, Inc.
- Neuronax SA
- Novartis AG
- Onward Medical
- Paradromics, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Acorda Therapeutics, Inc.
- Asterias Biotherapeutics, Inc.
- Athersys, Inc.
- Axoltis Pharma
- BioArctic AB
- BioAxone BioSciences, Inc.
- BioTime, Inc.
- Geron Corporation
- Histocell S.L.
- InVivo Therapeutics Holdings Corp
- Kringle Pharma, Inc.
- Lineage Cell Therapeutics, Inc.
- Merz Therapeutics
- Mitsubishi Tanabe Pharma Corp.
- Neuralstem, Inc.
- Neuronax SA
- Novartis AG
- Onward Medical
- Paradromics, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 274 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
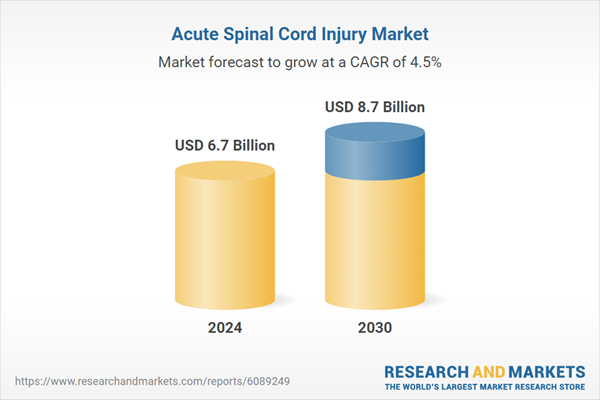
| Estimated Market Value ( USD | $ 6.7 Billion |
| Forecasted Market Value ( USD | $ 8.7 Billion |
| Compound Annual Growth Rate | 4.5% |
| Regions Covered | Global |