Global Pharmaceutical Water Market - Key Trends & Drivers Summarized
Why Is Pharmaceutical Water Becoming More Central in the Age of Complex Drug Manufacturing?
Pharmaceutical-grade water plays a critical and often underestimated role in drug manufacturing, directly influencing product safety, efficacy, and compliance. Used as a raw material, ingredient, and cleaning agent, pharmaceutical water comes in multiple purity grades - including Purified Water (PW), Water for Injection (WFI), and Highly Purified Water (HPW) - each tailored to specific pharmaceutical processes and dosage forms. As the complexity of biologics, sterile injectables, and personalized medicines increases, the demand for ultra-pure, contaminant-free water systems is rising across manufacturing facilities globally.The importance of pharmaceutical water extends to both upstream and downstream operations. In upstream biologics processing, it is essential for media preparation and buffer solutions, while in downstream purification and formulation, it serves as a solvent and diluent. Any deviation from regulatory water quality standards - set by the USP, EP, JP, or WHO - can compromise batch integrity, trigger product recalls, or result in regulatory action. As global pharmaceutical production scales and diversifies, the need for high-efficiency water purification, storage, and distribution systems has never been more critical.
How Are Innovations in Water System Engineering and Regulatory Harmonization Enhancing Market Demand?
Technological advancements in water purification are enabling the development of compact, energy-efficient, and fully automated water systems capable of delivering consistent quality in real time. Modern systems employ a multi-barrier approach using reverse osmosis (RO), ultrafiltration (UF), electrodeionization (EDI), and UV disinfection - integrated with real-time monitoring for microbial and chemical contaminants. The rise of hot water sanitizable and ozone-based cold sanitization units is eliminating the reliance on chemical sanitizers, improving operational safety and reducing downtime.Regulatory bodies are also harmonizing global guidelines, easing the acceptance of alternative technologies. Notably, the FDA and European Pharmacopoeia now permit the generation of WFI through non-distillation methods such as RO and EDI, provided quality and validation criteria are met. This shift is fostering innovation while reducing the environmental footprint of water-for-injection production. Continuous process verification and electronic data logging are further reinforcing compliance in aseptic and GMP environments where pharmaceutical water quality must be validated and controlled without interruption.
Why Are Emerging Markets, Biologics Manufacturing, and Sustainability Objectives Transforming Supply and Usage Patterns?
The expansion of pharmaceutical manufacturing hubs in Asia-Pacific, the Middle East, and Latin America is creating a robust demand for modular, scalable, and validated pharmaceutical water systems. These regions are seeing an influx of new manufacturing facilities driven by local drug access initiatives, contract manufacturing partnerships, and favorable regulatory incentives. As sterile injectables, biosimilars, and cell therapies become central to these expansions, the role of WFI and HPW systems becomes indispensable.Sustainability goals are also pushing manufacturers to rethink their water usage strategies. Companies are investing in closed-loop systems, wastewater recovery, and smart water management platforms that reduce environmental impact while optimizing cost. AI-enabled monitoring systems are being deployed to detect anomalies, predict maintenance needs, and reduce energy consumption. These green initiatives align with ESG frameworks and are helping pharmaceutical companies meet water conservation targets without compromising quality or compliance.
What's Driving the Global Growth of the Pharmaceutical Water Market?
The growth in the pharmaceutical water market is driven by several factors including the increasing demand for sterile manufacturing, the expansion of biopharmaceutical production, and the global shift toward automated and sustainable water systems. A major growth driver is the regulatory requirement for consistent, validated water purity across all manufacturing stages - from ingredient formulation to equipment cleaning.As drug complexity increases and aseptic manufacturing becomes mainstream, pharmaceutical companies are upgrading legacy water systems and adopting modular purification units that ensure flexibility, scalability, and regulatory readiness. The intersection of digitalization, automation, and sustainability is transforming pharmaceutical water from a utility function into a strategic asset. With growing regulatory convergence, infrastructure development in emerging markets, and the rise of advanced therapeutic manufacturing, the global pharmaceutical water market is set to see sustained and significant growth in the years ahead.
Report Scope
The report analyzes the Pharmaceutical Water market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Highly Purified Water, Water for Injection, Water for Inhalation, Deionized Water, Distilled Water, Other Product Types); Application (Drug Formulation, Cleaning & Purification, Cell Culture Production, Equipment Cleaning, Buffers & Solutions, Other Applications); End-User (Pharma & Biotech Companies, Contract Research & Manufacturing Organizations, Academics & Research Laboratories, Hospitals & Healthcare Facilities, Diagnostic Laboratories, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Highly Purified Water segment, which is expected to reach US$3 Billion by 2030 with a CAGR of a 8.2%. The Water for Injection segment is also set to grow at 4.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.8 Billion in 2024, and China, forecasted to grow at an impressive 10.1% CAGR to reach $2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pharmaceutical Water Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pharmaceutical Water Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pharmaceutical Water Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Pharmaceutical Water market report include:
- Alfa Laval
- Aqua Solutions, Inc.
- B. Braun Melsungen AG
- Baxter International Inc.
- BWT AG
- CovaChem, LLC
- Cytiva (Danaher Corporation)
- Danaher Corporation
- Dow Inc.
- Evoqua Water Technologies LLC
- Fresenius Kabi AG
- FUJIFILM Irvine Scientific
- General Electric
- Hikma Pharmaceuticals PLC
- Intermountain Life Sciences
- Lenntech B.V.
- Merck KGaA
- Nexus Pharmaceuticals, LLC
- Sartorius AG
- Veolia Water Technologies & Solutions
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alfa Laval
- Aqua Solutions, Inc.
- B. Braun Melsungen AG
- Baxter International Inc.
- BWT AG
- CovaChem, LLC
- Cytiva (Danaher Corporation)
- Danaher Corporation
- Dow Inc.
- Evoqua Water Technologies LLC
- Fresenius Kabi AG
- FUJIFILM Irvine Scientific
- General Electric
- Hikma Pharmaceuticals PLC
- Intermountain Life Sciences
- Lenntech B.V.
- Merck KGaA
- Nexus Pharmaceuticals, LLC
- Sartorius AG
- Veolia Water Technologies & Solutions
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 407 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
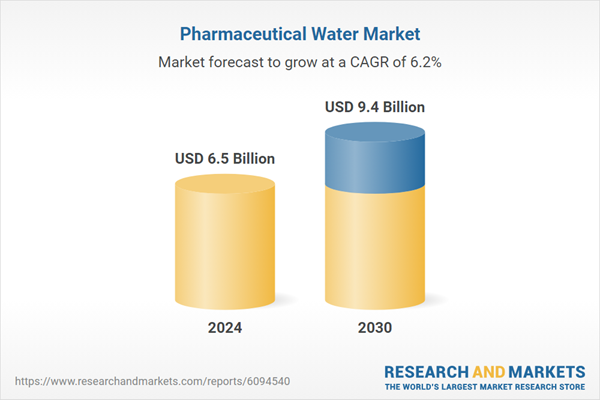
| Estimated Market Value ( USD | $ 6.5 Billion |
| Forecasted Market Value ( USD | $ 9.4 Billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |