Global Pertussis Vaccines Market - Key Trends & Drivers Summarized
Why Is Pertussis Vaccination Regaining Urgency in Global Immunization Strategies?
Pertussis, commonly known as whooping cough, remains a significant public health threat despite decades of immunization programs. Caused by Bordetella pertussis, the disease poses severe risks to infants and immunocompromised individuals, often leading to hospitalization or fatal respiratory complications. The resurgence of pertussis in both high- and low-income countries is driving renewed focus on vaccine coverage, booster programs, and maternal immunization strategies.The evolving epidemiology of pertussis - marked by increased incidence among adolescents and adults - has prompted healthcare agencies to revise immunization schedules. Recommendations now include adolescent boosters (Tdap), cocooning strategies for caregivers, and immunization during pregnancy to confer passive immunity to neonates. These policy shifts are supporting demand for both whole-cell (wP) and acellular (aP) pertussis vaccines, each with differing profiles of efficacy, reactogenicity, and public acceptance.
How Are Innovation and Reformulation Efforts Addressing Waning Immunity and Outbreak Management?
A major challenge in pertussis control is the waning immunity associated with acellular vaccines, which, although safer than whole-cell counterparts, provide shorter duration of protection. This has led to outbreaks even in highly vaccinated populations. Researchers and manufacturers are actively working on next-generation pertussis vaccines with improved adjuvants, extended immunogenicity, and broader strain coverage.Novel formulations under development include live attenuated nasal vaccines, genetically detoxified pertussis toxins, and nanoparticle-based adjuvants aimed at enhancing mucosal immunity. These innovations are intended to strengthen herd immunity, especially in populations with high transmission potential such as school-aged children and healthcare workers. Additionally, advancements in combination vaccines (e.g., DTaP, Tdap-IPV-HepB-Hib) are streamlining immunization logistics and improving compliance in pediatric and adult populations.
Why Are Global Health Initiatives, Supply Chain Enhancements, and Policy Frameworks Vital to Market Expansion?
Global vaccination efforts led by organizations such as Gavi, WHO, and UNICEF continue to play a pivotal role in expanding pertussis vaccine access in low- and middle-income countries. These agencies support procurement, distribution, and cold-chain management while also funding education campaigns to reduce vaccine hesitancy. Public-private partnerships are further aiding vaccine affordability and localized production through technology transfers and licensing agreements.Policy frameworks are also evolving to incorporate pertussis surveillance into national disease monitoring systems. Integrated immunization schedules, digital tracking tools, and catch-up campaigns are being implemented to address immunity gaps. Countries are increasingly prioritizing pertussis control within maternal and child health programs, boosting both demand and funding for vaccines. These systemic developments are improving market predictability and encouraging long-term investment by biopharmaceutical companies.
What's Driving the Continued Growth of the Pertussis Vaccines Market Globally?
The growth in the pertussis vaccines market is driven by several factors including rising disease incidence, expansion of maternal immunization programs, and technological innovations in vaccine development. A key growth driver is the shift toward booster-based lifelong immunization strategies that extend coverage beyond infancy and target adolescents, adults, and seniors.Moreover, the rising awareness of pertussis complications, improvements in diagnostic surveillance, and increased integration with combination vaccine platforms are further strengthening market momentum. As countries scale up efforts to meet global immunization targets and mitigate preventable deaths, pertussis vaccines remain central to national and international public health agendas.
With the ongoing emergence of pertussis variants, increasing healthcare investments in immunization infrastructure, and strong demand for safer, more effective vaccine formulations, the pertussis vaccines market is positioned for sustained global growth in both public health and private clinical sectors.
Report Scope
The report analyzes the Pertussis Vaccines market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (DTaP Vaccine, Tdap Vaccine); End-Use (Vaccination Centers, Hospitals, Clinics, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the DTaP Vaccine segment, which is expected to reach US$5.7 Billion by 2030 with a CAGR of a 5.2%. The Tdap Vaccine segment is also set to grow at 3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.7 Billion in 2024, and China, forecasted to grow at an impressive 8.3% CAGR to reach $1.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pertussis Vaccines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pertussis Vaccines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pertussis Vaccines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 23andMe, AltheaDx, Inc., Arcara Personalized Psychiatry, BrainsWay Ltd., BrightQuest Treatment Centers and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Pertussis Vaccines market report include:
- Astellas Pharma Inc.
- Bharat Biotech International Limited
- Biological E. Limited
- BioNet-Asia Co., Ltd.
- Cadila Healthcare Ltd. (Zydus Cadila)
- Chiron Behring Vaccines Pvt. Ltd.
- CSL Limited (Seqirus)
- GlaxoSmithKline plc
- Green Cross Corporation
- Haffkine Bio-Pharmaceutical Corporation Ltd.
- ILiAD Biotechnologies, LLC
- Indian Immunologicals Ltd.
- Merck & Co., Inc.
- Panacea Biotec Ltd.
- Pfizer Inc.
- Sanofi Pasteur
- Serum Institute of India Pvt. Ltd.
- Shanta Biotechnics Pvt. Ltd.
- Sinovac Biotech Ltd.
- Wuhan Institute of Biological Products Co., Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Astellas Pharma Inc.
- Bharat Biotech International Limited
- Biological E. Limited
- BioNet-Asia Co., Ltd.
- Cadila Healthcare Ltd. (Zydus Cadila)
- Chiron Behring Vaccines Pvt. Ltd.
- CSL Limited (Seqirus)
- GlaxoSmithKline plc
- Green Cross Corporation
- Haffkine Bio-Pharmaceutical Corporation Ltd.
- ILiAD Biotechnologies, LLC
- Indian Immunologicals Ltd.
- Merck & Co., Inc.
- Panacea Biotec Ltd.
- Pfizer Inc.
- Sanofi Pasteur
- Serum Institute of India Pvt. Ltd.
- Shanta Biotechnics Pvt. Ltd.
- Sinovac Biotech Ltd.
- Wuhan Institute of Biological Products Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 285 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
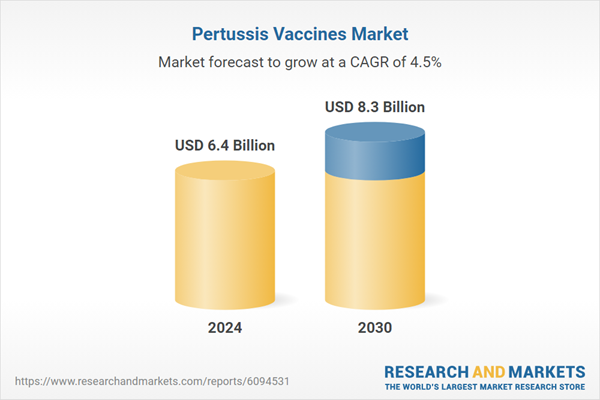
| Estimated Market Value ( USD | $ 6.4 Billion |
| Forecasted Market Value ( USD | $ 8.3 Billion |
| Compound Annual Growth Rate | 4.5% |
| Regions Covered | Global |