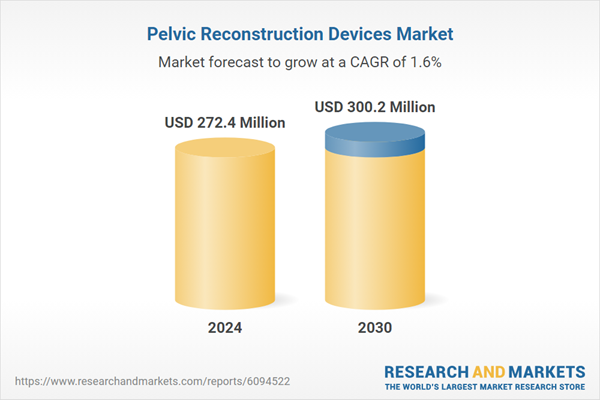
Global Pelvic Reconstruction Devices Market - Key Trends & Drivers Summarized
Why Is the Demand for Pelvic Reconstruction Devices Growing Amid Demographic Shifts and Rising Awareness?
Pelvic reconstruction devices are critical medical implants and tools used in the surgical treatment of pelvic organ prolapse (POP), pelvic fractures, and other pelvic floor disorders. With the rising incidence of these conditions - particularly among postmenopausal women, trauma patients, and individuals undergoing cancer-related pelvic surgeries - the global demand for pelvic reconstruction solutions is accelerating. Aging populations, especially in developed regions, are driving procedural volumes as the risk of pelvic floor dysfunction significantly increases with age, multiparity, and hormonal changes.Greater awareness of pelvic health among both patients and clinicians is reducing the stigma around pelvic floor disorders and increasing demand for surgical intervention. Minimally invasive techniques, such as laparoscopic and robotic-assisted sacrocolpopexy, are gaining preference over traditional open surgeries due to lower complication rates and faster recovery. These shifts are influencing the adoption of newer device types, including mesh implants, biologic grafts, bone plates, fixation screws, and tissue anchors - all designed to improve long-term outcomes and restore pelvic integrity.
How Are Material Advancements, Regulatory Oversight, and Surgical Innovations Shaping the Market Landscape?
The evolution of materials used in pelvic reconstruction has been a focal point of product innovation and regulatory scrutiny. Synthetic meshes, once the dominant choice, have come under regulatory pressure following reports of erosion and pain. As a result, the industry is pivoting toward lighter, monofilament meshes, absorbable materials, and biologically derived grafts that offer better biocompatibility and fewer adverse events. Manufacturers are now investing in mesh designs with macroporous structures and anti-microbial coatings to reduce infection and fibrosis risks.On the procedural front, robotic-assisted surgeries are enabling higher precision in pelvic reconstruction, particularly in complex anatomical repairs and deep pelvic procedures. Innovations in 3D imaging, intraoperative navigation, and sutureless fixation are also streamlining surgical workflows and improving anatomical alignment. Regulatory bodies such as the U.S. FDA and European CE mark authorities are tightening pre-market approval and post-market surveillance requirements, influencing device development timelines and raising the barrier to market entry.
Why Are Gender-Specific Applications and Orthopedic-Trauma Integration Creating New Growth Pathways?
While pelvic floor reconstruction is more commonly associated with women's health, the market for pelvic fracture fixation devices in orthopedic and trauma surgery is growing among both genders. High-impact injuries from road accidents, falls, and sports are increasing, particularly among aging populations with fragile bones and younger individuals with active lifestyles. This dual demand is driving product development in areas such as bioresorbable plates, modular sacral screws, and internal pelvic fixation systems that can accommodate diverse anatomical and surgical needs.In urogynecology, gender-specific implants designed for anterior, posterior, and apical compartment prolapse are gaining traction. These devices are tailored for better anatomical fit, lower migration risk, and more intuitive implantation. Customizable kits that combine fixation systems with biologic mesh or patient-specific 3D-printed components are becoming popular among high-volume pelvic reconstructive surgeons. This personalization of pelvic surgery, driven by advanced preoperative planning and simulation, is broadening the scope of reconstructive options available to patients.
What's Driving the Growth of the Pelvic Reconstruction Devices Market Worldwide?
The growth in the pelvic reconstruction devices market is driven by several factors including the increasing global burden of pelvic organ prolapse, trauma-related pelvic fractures, and the expanding adoption of minimally invasive surgical techniques. A key growth driver is the combination of clinical need and technological innovation - enabling more precise, patient-friendly, and outcome-optimized reconstructive procedures.Reimbursement reforms, growing surgical training networks, and rising investment in women's health by medtech firms are accelerating the adoption of next-generation pelvic devices. Additionally, public health initiatives that encourage early diagnosis of pelvic floor disorders and trauma-related injury treatment are strengthening procedural demand. With aging demographics, surgical standardization, and technology convergence aligning globally, the pelvic reconstruction devices market is poised for sustained innovation and growth.
Report Scope
The report analyzes the Pelvic Reconstruction Devices market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Vaginal Mesh, Biological Mesh, Synthetic Mesh, Vaginal Sling Systems, Vaginal Pessary); Indication (Pelvic Organ Prolapse, Vaginal Prolapse, Rectal Prolapse, Urinary Incontinence, Interstitial Cystitis, Fistulae); End-Use (Hospitals, Ambulatory Surgery Centers, Specialty Clinics, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Vaginal Mesh segment, which is expected to reach US$99.4 Million by 2030 with a CAGR of a 1%. The Biological Mesh segment is also set to grow at 2.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $74.2 Million in 2024, and China, forecasted to grow at an impressive 3% CAGR to reach $54.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pelvic Reconstruction Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pelvic Reconstruction Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pelvic Reconstruction Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 3M, AGC Inc., ASML Holding N.V., Canatu, Dai Nippon Printing Co., Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 39 companies featured in this Pelvic Reconstruction Devices market report include:
- Betatech Medical
- Boston Scientific Corporation
- Caldera Medical
- Coloplast A/S
- Cook Medical
- CooperSurgical Inc.
- Dipromed Srl
- Ethicon (Johnson & Johnson)
- Integra LifeSciences
- MedGyn Products Inc.
- Medtronic plc
- Neomedic International
- Panpac Medical Corporation
- Promedon
- Serag-Wiessner GmbH & Co. KG
- Smith & Nephew plc
- Stryker Corporation
- TEI Biosciences Inc.
- Zephyr Surgical Implants
- Zimmer Biomet Holdings, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Betatech Medical
- Boston Scientific Corporation
- Caldera Medical
- Coloplast A/S
- Cook Medical
- CooperSurgical Inc.
- Dipromed Srl
- Ethicon (Johnson & Johnson)
- Integra LifeSciences
- MedGyn Products Inc.
- Medtronic plc
- Neomedic International
- Panpac Medical Corporation
- Promedon
- Serag-Wiessner GmbH & Co. KG
- Smith & Nephew plc
- Stryker Corporation
- TEI Biosciences Inc.
- Zephyr Surgical Implants
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 390 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 272.4 Million |
| Forecasted Market Value ( USD | $ 300.2 Million |
| Compound Annual Growth Rate | 1.6% |
| Regions Covered | Global |