Global Hopped Malt Extracts Market - Key Trends & Drivers Summarized
Why Are Hopped Malt Extracts Gaining Traction Among Modern Brewers and Food Manufacturers?
Hopped malt extracts are becoming increasingly valuable in both traditional and craft brewing markets due to their ability to simplify production, reduce brewing time, and ensure flavor consistency. These extracts are concentrated solutions of malted barley infused with hop bitterness and aroma, allowing brewers to skip several steps of the brewing process while retaining the characteristic depth and complexity of hop-forward beer profiles. They offer a shelf-stable, cost-effective alternative to raw malt and hops, making them ideal for microbreweries, homebrewers, and commercial beverage manufacturers seeking scalable production without quality trade-offs.The rise in small-scale brewing operations globally, particularly in North America, Europe, and parts of Asia, is creating demand for ready-to-use ingredients that minimize capital expenditure and technical bottlenecks. Additionally, the increasing popularity of hop-centric beer styles such as IPAs, pale ales, and hoppy lagers is fueling the need for flexible, flavor-specific malt extract formulations. These extracts are also gaining use outside of brewing - in food, sauces, and nutritional products - due to their natural origin, deep umami flavor, and potential as a clean-label additive.
How Are Production Techniques and Customization Capabilities Evolving?
Advancements in extract processing technologies, including vacuum evaporation and low-temperature concentration, are preserving the delicate aroma compounds and volatile hop oils during malt extract production. Manufacturers are refining the balance between bitterness units (IBUs), malt color, and aromatic profiles to cater to specific beer styles. By adjusting hop timing, malt composition, and wort boiling conditions during extract creation, producers can tailor the final product for lagers, ales, stouts, and specialty brews.Customization is a key value driver, with suppliers offering extracts with different hop varieties (Cascade, Citra, Saaz, etc.), malt bases (Pilsner, Vienna, Munich), and concentrations. Packaging innovations - such as aseptic pouches, drums, and bag-in-box systems - are improving storage and handling, especially for bulk users. Integration with digital brewing platforms and dosing equipment is also simplifying ingredient management for larger breweries and contract brewers. These innovations are elevating hopped malt extracts from convenience products to integral, quality-focused components in premium beer production.
Where Is Demand Growing Beyond the Traditional Brewing Segment?
While the brewing industry remains the dominant consumer, demand for hopped malt extracts is expanding into non-alcoholic beverages, functional foods, and culinary products. As non-alcoholic craft beer and hop-infused seltzers grow in popularity, producers are using malt extracts to add body, mouthfeel, and bitterness without fermentation. Health-oriented beverages are incorporating malt extracts for natural sweetness and B-vitamin content. In the culinary world, hopped malt extracts are being used in marinades, dressings, sauces, and even plant-based meat formulations for their savory, umami-enhancing characteristics.Geographically, demand is rising in emerging craft beer markets across Southeast Asia, Latin America, and Eastern Europe, where breweries are looking for standardized ingredients to maintain quality during scale-up. E-commerce growth in homebrewing kits is also boosting extract sales in consumer markets. Food and beverage formulators targeting clean-label, naturally flavored, and plant-derived ingredients are turning to malt extracts as both functional components and marketing differentiators. These diverse applications are making hopped malt extracts more than just a brewer's tool - they're becoming versatile ingredients across the food and beverage value chain.
The Growth in the Hopped Malt Extracts Market Is Driven by Several Factors…
It is primarily driven by the global expansion of craft and home brewing, the growing demand for convenience in beer production, and the increasing appeal of natural, label-friendly food ingredients. As brewers seek to optimize efficiency while maintaining flavor complexity, malt extracts offer consistent quality, reduced labor, and lower equipment requirements. Additionally, the booming market for non-alcoholic and hybrid beverages is fostering creative applications for hopped extracts beyond alcohol.The clean-label movement in the food industry is promoting the use of minimally processed, naturally derived ingredients like malt extract as sweeteners, flavor enhancers, and texturizers. Product development in gluten-free and vegan categories is also leveraging malt-based syrups for flavor masking and nutritional enhancement. On the supply side, advancements in extraction and packaging are enhancing shelf life, transportability, and customization. Collectively, these trends are solidifying hopped malt extracts as an adaptable and growing segment across both brewing and broader food manufacturing ecosystems.
Report Scope
The report analyzes the Hopped Malt Extracts market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Source (Barley, Wheat, Rye, Other Sources); Form (Liquid, Dry, Other Forms); Distribution Channel (B2B, B2C, Online, Specialty Stores, Other Distribution Channels); Application (Beer, Whisky, Food, Pharma, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Barley Source segment, which is expected to reach US$1 Billion by 2030 with a CAGR of a 6.8%. The Wheat Source segment is also set to grow at 4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $381.1 Million in 2024, and China, forecasted to grow at an impressive 9.1% CAGR to reach $395.9 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Hopped Malt Extracts Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Hopped Malt Extracts Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Hopped Malt Extracts Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 3M, APLIX, Clarendon Specialty Fasteners, DirecTex, Dunlap Industries Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Hopped Malt Extracts market report include:
- Bairds Malt Ltd.
- BarthHaas
- BrewDemon
- Brewferm
- Briess Malt & Ingredients Co.
- CereX B.V.
- Charles Faram & Co. Ltd.
- Coopers Brewery
- Crisp Malting Group
- Crosby Hop Farm LLC
- Hambleton Bard Ltd.
- Hopco Pty Ltd.
- Hopsteiner
- Malteurop Groupe
- Mangrove Jack's
- Muntons plc
- P.A.B. srl - Mr. Malt
- Rahr Malting Co.
- Simpsons Malt Ltd.
- Weyermann Specialty Malts
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Bairds Malt Ltd.
- BarthHaas
- BrewDemon
- Brewferm
- Briess Malt & Ingredients Co.
- CereX B.V.
- Charles Faram & Co. Ltd.
- Coopers Brewery
- Crisp Malting Group
- Crosby Hop Farm LLC
- Hambleton Bard Ltd.
- Hopco Pty Ltd.
- Hopsteiner
- Malteurop Groupe
- Mangrove Jack's
- Muntons plc
- P.A.B. srl - Mr. Malt
- Rahr Malting Co.
- Simpsons Malt Ltd.
- Weyermann Specialty Malts
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 486 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
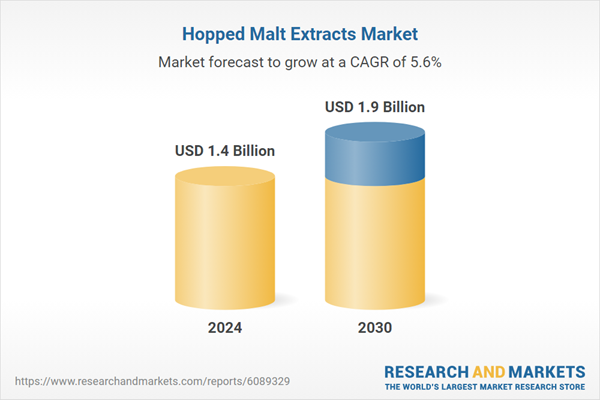
| Estimated Market Value ( USD | $ 1.4 Billion |
| Forecasted Market Value ( USD | $ 1.9 Billion |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |