Global Leukemia Screening Market - Key Trends & Drivers Summarized
Why Is Early Screening Becoming Central to Leukemia Management?
Leukemia, a group of hematologic malignancies affecting white blood cells, continues to impose a significant global health burden, especially due to its rapid onset and variable presentation. Early and accurate screening is vital in improving prognosis, enabling timely therapeutic interventions, and tailoring treatment strategies. With survival rates strongly linked to stage at diagnosis, healthcare systems worldwide are prioritizing leukemia screening as part of broader oncology early-detection programs. Screening is particularly critical for high-risk populations, including individuals with genetic predispositions, prior exposure to radiation or chemotherapy, and those with certain pre-leukemic conditions like myelodysplastic syndromes.Technological advancements are reshaping leukemia detection methods. Traditional complete blood count (CBC) and peripheral smear tests are being augmented by sophisticated molecular diagnostics and flow cytometry techniques, which enable the detection of minimal residual disease (MRD) and early-stage mutations. These tools are enhancing clinical decision-making, especially in distinguishing between leukemia subtypes such as acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), and acute lymphoblastic leukemia (ALL). As precision medicine becomes a standard in oncology, leukemia screening is moving beyond simple detection to encompass disease monitoring and risk stratification.
How Are Diagnostic Technologies Evolving to Enable Precision and Accessibility?
Innovations in molecular diagnostics, liquid biopsy, and genomics are significantly enhancing the precision of leukemia screening. Next-generation sequencing (NGS) is now widely used to identify mutations in leukemia-associated genes such as FLT3, NPM1, and BCR-ABL, allowing for subtype classification and targeted therapy selection. Multiplex PCR assays and digital droplet PCR are also being employed to detect fusion genes and low-frequency mutations that are otherwise difficult to identify through conventional methods. These technologies are enabling clinicians to catch leukemia in its earliest stages - often before symptoms manifest - and monitor treatment response with high sensitivity.In parallel, point-of-care (POC) testing tools are being developed to decentralize screening and improve access, particularly in low-resource settings where laboratory infrastructure may be limited. Mobile diagnostic platforms using microfluidic devices, lab-on-a-chip technologies, and AI-driven interpretation are being tested to support mass screening programs. These tools, combined with electronic health record (EHR) integration and cloud-based data analytics, are streamlining leukemia detection workflows and enabling broader implementation of population-level surveillance.
What Trends Are Influencing Clinical Adoption and Public Health Strategy?
Clinical and public health strategies are increasingly emphasizing risk-based and age-targeted leukemia screening. Pediatric leukemia, particularly ALL, has seen significant survival improvements due to integrated screening and early intervention, but adult leukemia remains a diagnostic challenge due to nonspecific symptoms and the absence of routine hematologic checks. National cancer control programs in several countries are incorporating hematologic malignancy screening into broader NCD prevention frameworks, especially where environmental or occupational risk factors are high.Insurance coverage for advanced diagnostics and growing investment in oncology infrastructure are also shaping market growth. Governments and nonprofit organizations are investing in diagnostic capacity building, genetic counseling programs, and awareness campaigns to promote early testing. Additionally, hematology-oncology research collaborations are fueling biomarker discovery and accelerating the clinical translation of novel screening modalities. These trends are helping to reduce diagnostic delays and support the move toward personalized leukemia care.
What Is Driving Growth in the Global Leukemia Screening Market?
The growth in the leukemia screening market is driven by several factors, including advances in molecular diagnostics, increasing leukemia incidence, and expanding precision medicine frameworks. Rising demand for early detection tools, particularly among aging populations and genetically predisposed individuals, is supporting widespread screening initiatives. The availability of highly sensitive tests such as flow cytometry and NGS is enabling earlier diagnosis, risk profiling, and disease monitoring, while liquid biopsy is emerging as a non-invasive and scalable option for repeat assessments.Policy shifts toward universal cancer screening, rising healthcare expenditures, and improved public awareness are expanding market access across both developed and emerging economies. Furthermore, the integration of AI and digital health tools is accelerating diagnostic throughput and enabling population-wide implementation of screening programs. As clinical guidelines evolve to recommend molecular profiling and genetic screening as part of routine oncology care, the global leukemia screening market is poised for sustained expansion, driven by both technological innovation and the urgent need for earlier, more precise cancer detection.
Report Scope
The report analyzes the Leukemia Screening market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Test Type (Complete Blood Count, Bone Marrow Aspiration & Biopsy, Flow Cytometry, Cytogenetic Analysis, Polymerase Chain Reaction, Immunophenotyping, Other Test Types); Disease Type (Acute Lymphoblastic Leukemia, Acute Myeloid Leukemia, Chronic Lymphocytic Leukemia, Chronic Myeloid Leukemia, Other Disease Types); Age Group (Pediatric, Adult, Geriatric); End-Use (Hospitals & Clinics, Diagnostic Laboratories, Research Institutes, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Complete Blood Count segment, which is expected to reach US$2.7 Billion by 2030 with a CAGR of a 5.9%. The Bone Marrow Aspiration & Biopsy segment is also set to grow at 4.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.1 Billion in 2024, and China, forecasted to grow at an impressive 8.9% CAGR to reach $2.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Leukemia Screening Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Leukemia Screening Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Leukemia Screening Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AIDP, Inc., AnaSpec, Inc., Asahi Group Holdings, Ltd., Biotech Pharmacal, Blue California and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 37 companies featured in this Leukemia Screening market report include:
- Abbott Laboratories
- Adaptive Biotechnologies
- Alercell
- ArcherDX
- Asuragen (Bio-Techne)
- Beckman Coulter
- Bio-Rad Laboratories
- Danaher Corporation
- DiaSorin
- Exact Sciences
- F. Hoffmann-La Roche Ltd
- Grail
- Guardant Health
- Illumina
- Invivoscribe
- Natera
- Prenetics
- QIAGEN
- Quest Diagnostics
- SkylineDx
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Adaptive Biotechnologies
- Alercell
- ArcherDX
- Asuragen (Bio-Techne)
- Beckman Coulter
- Bio-Rad Laboratories
- Danaher Corporation
- DiaSorin
- Exact Sciences
- F. Hoffmann-La Roche Ltd
- Grail
- Guardant Health
- Illumina
- Invivoscribe
- Natera
- Prenetics
- QIAGEN
- Quest Diagnostics
- SkylineDx
Table Information
| Report Attribute | Details |
|---|---|
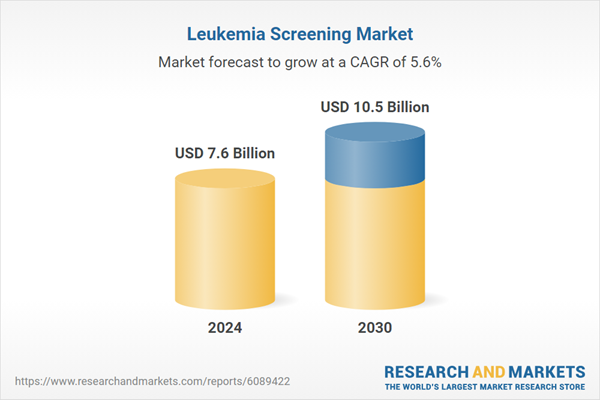
| No. of Pages | 487 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 7.6 Billion |
| Forecasted Market Value ( USD | $ 10.5 Billion |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |