Global Leptospirosis Market - Key Trends & Drivers Summarized
Why Is Leptospirosis Emerging as a Global Public Health Concern?
Leptospirosis, a bacterial zoonotic disease caused by Leptospira species, is gaining renewed attention as outbreaks increase in both tropical and temperate regions. The disease is transmitted through direct contact with the urine of infected animals or contaminated water and soil, making it especially prevalent in areas with poor sanitation, flooding, and high rodent populations. As climate change leads to more extreme weather events, including hurricanes and heavy rainfall, the incidence of leptospirosis is rising in urban and peri-urban regions, where waterborne exposure is common. This dynamic has turned what was once seen as a rural or occupational disease into a broader public health challenge.In many low- and middle-income countries, leptospirosis remains underdiagnosed due to its nonspecific symptoms, which mimic viral infections such as dengue and influenza. However, growing awareness and improved diagnostic tools - particularly point-of-care molecular tests - are facilitating earlier identification and intervention. Public health agencies are now integrating leptospirosis surveillance into broader infectious disease monitoring systems, particularly in Southeast Asia, Latin America, and sub-Saharan Africa. These efforts are helping to map outbreaks, understand seasonal patterns, and identify high-risk populations for targeted control strategies.
How Are Diagnostics, Therapeutics, and Preventive Strategies Evolving?
Advances in diagnostic methods are significantly improving the clinical management of leptospirosis. Traditional tests such as the microscopic agglutination test (MAT) are being supplemented by faster, more sensitive techniques including PCR, ELISA, and lateral flow assays. These innovations are enabling earlier intervention with antibiotics such as doxycycline and penicillin, which are most effective in the early phase of infection. Combination therapies and supportive care approaches are being refined, especially for severe cases involving liver or kidney dysfunction.On the preventive front, human and veterinary vaccines are under renewed development. Existing animal vaccines are being improved to offer broader serovar coverage, while human vaccine candidates are in the pipeline with novel formulations aimed at providing cross-protective immunity. Integrated vector control strategies, sanitation improvements, and rodent population management are also being prioritized in endemic regions. Additionally, the rise of public-private partnerships and One Health approaches - linking human, animal, and environmental health - is enhancing long-term prevention and response planning.
What Are the Emerging Trends in Surveillance, Outbreak Management, and Public Policy?
The COVID-19 pandemic has accelerated investment in public health infrastructure, which is now being leveraged to track other infectious diseases, including leptospirosis. Digital surveillance systems, mobile reporting platforms, and GIS mapping tools are being used to track outbreaks in real time and forecast future hotspots based on weather patterns and socio-environmental factors. Governments are incorporating leptospirosis risk into disaster response frameworks, particularly in flood-prone regions where post-disaster outbreaks are frequent.International agencies such as WHO and CDC are also enhancing support for leptospirosis research and control efforts through funding, training, and coordinated guidelines. Policy frameworks promoting One Health collaboration between ministries of health, agriculture, and environment are gaining traction, especially in countries where livestock and wildlife play a major role in disease transmission. These trends are creating a more structured and integrated approach to managing leptospirosis as a global health priority.
What Is Driving Growth in the Leptospirosis Diagnostics and Treatment Market Globally?
The growth in the leptospirosis market is driven by several factors including climate-induced disease spread, improved diagnostics, and heightened public health investment. A key driver is the increase in flood-related outbreaks in urban areas, creating strong demand for rapid testing, early treatment protocols, and stockpiling of antibiotics. Rising pet ownership and livestock farming in developing nations are also expanding the reservoir of potential infections, further boosting veterinary diagnostics and vaccine markets.Government-backed health initiatives, coupled with donor funding from global health organizations, are increasing access to diagnostic kits and surveillance technologies in underserved regions. Advances in recombinant vaccine development and bioinformatics-based serovar tracking are attracting research investments, while pharma companies are exploring repurposed drugs and immune-modulating therapies. Lastly, the integration of leptospirosis monitoring into pandemic preparedness frameworks is reinforcing its relevance in both human and veterinary health ecosystems - fueling growth in diagnostics, treatment, and public health services.
Report Scope
The report analyzes the Leptospirosis market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Diagnosis (Complete Blood Count, Creatine Kinase, Liver Enzymes, Urinalysis, Other Diagnosis); Administration Route (Oral, Parenteral, Intravenous); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Complete Blood Count segment, which is expected to reach US$193.2 Million by 2030 with a CAGR of a 3.7%. The Creatine Kinase segment is also set to grow at 4.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $139 Million in 2024, and China, forecasted to grow at an impressive 7.5% CAGR to reach $132 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Leptospirosis Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Leptospirosis Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Leptospirosis Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abliva AB, Bayer AG, Biogen Inc., Edison Pharmaceuticals, Inc., GSK Plc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Leptospirosis market report include:
- Anatolia Geneworks
- Bayer AG
- Boehringer Ingelheim GmbH
- Ceva Santé Animale
- Elanco Animal Health
- IDEXX Laboratories, Inc.
- Indian Immunologicals Ltd.
- International Vaccine Institute (IVI)
- Merck & Co., Inc.
- MSD Animal Health
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Serum Institute of India
- Shionogi & Co., Ltd.
- Takeda Pharmaceutical Company
- Vaxxinova International B.V.
- Virbac
- Zoetis Inc.
- Zydus Cadila
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Anatolia Geneworks
- Bayer AG
- Boehringer Ingelheim GmbH
- Ceva Santé Animale
- Elanco Animal Health
- IDEXX Laboratories, Inc.
- Indian Immunologicals Ltd.
- International Vaccine Institute (IVI)
- Merck & Co., Inc.
- MSD Animal Health
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Serum Institute of India
- Shionogi & Co., Ltd.
- Takeda Pharmaceutical Company
- Vaxxinova International B.V.
- Virbac
- Zoetis Inc.
- Zydus Cadila
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 382 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
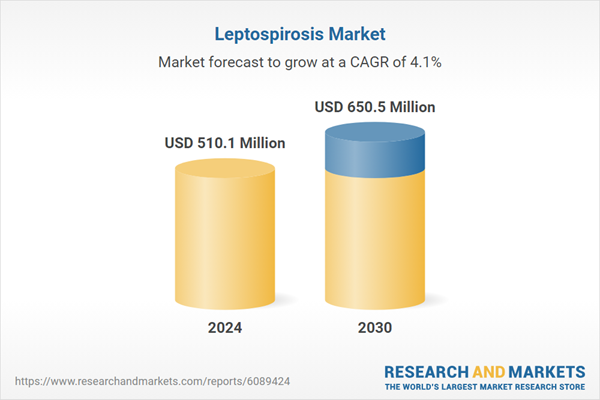
| Estimated Market Value ( USD | $ 510.1 Million |
| Forecasted Market Value ( USD | $ 650.5 Million |
| Compound Annual Growth Rate | 4.1% |
| Regions Covered | Global |