Global IV Fluid Monitoring Devices Market - Key Trends & Drivers Summarized
Why Are IV Fluid Monitoring Devices Vital in Modern Healthcare Delivery?
IV fluid monitoring devices have become essential tools in clinical environments to ensure accurate, timely, and safe administration of intravenous fluids to patients. These systems help healthcare providers maintain optimal fluid balance, detect infusion anomalies, and prevent complications such as fluid overload, under-infusion, or infiltration. In critical care, emergency medicine, surgical recovery, and pediatric settings, real-time monitoring of IV fluids is crucial for maintaining hemodynamic stability and supporting rapid therapeutic interventions.Traditional manual observation methods - such as watching drip chambers - are increasingly being replaced by automated, digital monitoring systems that provide continuous and precise tracking of infusion volumes, rates, and durations. These devices minimize human error, improve clinical efficiency, and enable better documentation of fluid therapy. With increasing complexity in patient conditions and rising expectations for safety and accountability in care delivery, IV fluid monitoring technologies are becoming standard in both acute and chronic care settings worldwide.
What Technological Advancements Are Transforming IV Fluid Monitoring Systems?
Technological innovation is rapidly enhancing the capabilities of IV fluid monitoring devices, transitioning them from basic flow sensors to integrated, intelligent systems. Advanced devices now use ultrasonic, pressure-based, or optical sensors to measure fluid flow with high accuracy without coming into direct contact with the IV line, reducing the risk of contamination. These non-invasive systems can detect blockages, air bubbles, infiltration, or leaks in real time and alert clinical staff immediately.Smart infusion systems and wireless-enabled monitoring platforms are enabling real-time data integration with electronic health records (EHRs), clinical dashboards, and remote monitoring systems. AI-powered algorithms are being embedded to provide predictive analytics, suggesting adjustments in infusion rates or preempting adverse events. Portable, battery-operated devices designed for ambulatory and home care are extending IV fluid monitoring to outpatient and post-acute settings. These innovations are supporting personalized fluid management, improving patient outcomes, and reducing caregiver workload across all healthcare environments.
Which Clinical Settings and Patient Populations Are Driving Adoption of These Devices?
Hospitals remain the largest users of IV fluid monitoring devices, especially in intensive care units (ICUs), surgical wards, and emergency departments, where fluid balance can shift rapidly and requires continuous oversight. Surgical patients, trauma victims, neonates, and individuals with renal or cardiac comorbidities are particularly reliant on precise fluid management. In pediatric care, where even slight deviations in fluid levels can be dangerous, digital monitoring provides essential precision and reassurance.Home healthcare and ambulatory infusion therapy centers are emerging as high-growth segments, especially as patients with chronic conditions such as cancer, gastrointestinal disorders, or infections are increasingly managed outside traditional hospital settings. These patients often require prolonged or intermittent infusions, which benefit from portable, intelligent monitoring solutions that ensure safe and accurate delivery. Moreover, long-term care facilities and hospices are adopting IV fluid monitoring devices to improve care standards and reduce hospital readmissions among elderly and high-risk populations.
The Growth in the IV Fluid Monitoring Devices Market Is Driven by Several Factors…
The growth in the IV fluid monitoring devices market is driven by several factors, including rising hospitalization rates, growing adoption of smart healthcare technologies, and the expanding use of IV therapy in outpatient and home-based settings. As global healthcare systems shift toward value-based care, there is increasing emphasis on precision, safety, and real-time responsiveness in fluid administration - areas where digital IV monitoring excels. Advances in miniaturization, wireless communication, and integration with digital health records are making these devices more versatile and scalable for different care environments.The increasing burden of chronic diseases, such as cancer, kidney failure, and sepsis, is also amplifying the need for reliable and continuous fluid management. Additionally, concerns over fluid-related medical errors, infusion-related adverse events, and nosocomial infections are prompting hospitals to invest in more advanced and automated monitoring systems. Regulatory guidelines and hospital accreditation standards are further encouraging adoption of smart infusion monitoring. As technology costs decline and device usability improves, IV fluid monitoring systems are becoming indispensable tools in enhancing clinical precision, operational efficiency, and patient safety across the continuum of care.
Report Scope
The report analyzes the IV Fluid Monitoring Devices market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Device Type (Desktop, Portable); End-Use (Hospitals & Clinics, Ambulatory Surgery Centers, Home Care, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Desktop Devices segment, which is expected to reach US$5 Billion by 2030 with a CAGR of a 7%. The Portable Devices segment is also set to grow at 3.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.4 Billion in 2024, and China, forecasted to grow at an impressive 9.8% CAGR to reach $1.5 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global IV Fluid Monitoring Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global IV Fluid Monitoring Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global IV Fluid Monitoring Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Accenture Security, Akamai Technologies, Alphabet Inc. (Google), Amazon Web Services (AWS), AT&T Cybersecurity and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this IV Fluid Monitoring Devices market report include:
- 3M Company
- Arcomed AG
- B. Braun Melsungen AG
- Baxter International Inc.
- BC Group International Inc.
- Becton, Dickinson and Company
- Cole-Parmer Instrument Company
- Datrend Systems Inc.
- EVELABS TECHNOLOGIES Pvt. Ltd.
- Fortive Corporation
- Gossen Metrawatt GmbH
- ICU Medical Inc.
- IRadimed Corporation
- ivWatch LLC
- Micrel Medical Devices SA
- MONIDOR Oy Ltd.
- NETECH Corp.
- Pentland Medical Ltd.
- Pronk Technologies Inc.
- Seaward Group USA
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3M Company
- Arcomed AG
- B. Braun Melsungen AG
- Baxter International Inc.
- BC Group International Inc.
- Becton, Dickinson and Company
- Cole-Parmer Instrument Company
- Datrend Systems Inc.
- EVELABS TECHNOLOGIES Pvt. Ltd.
- Fortive Corporation
- Gossen Metrawatt GmbH
- ICU Medical Inc.
- IRadimed Corporation
- ivWatch LLC
- Micrel Medical Devices SA
- MONIDOR Oy Ltd.
- NETECH Corp.
- Pentland Medical Ltd.
- Pronk Technologies Inc.
- Seaward Group USA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 269 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
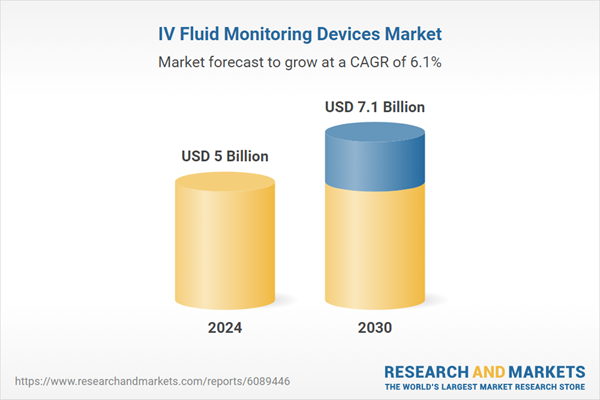
| Estimated Market Value ( USD | $ 5 Billion |
| Forecasted Market Value ( USD | $ 7.1 Billion |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |