Speak directly to the analyst to clarify any post sales queries you may have.
The Premature Ovarian Failure Cure Market is evolving rapidly as emerging therapies, advanced research, and streamlined regulatory pathways converge to drive innovation and address significant unmet needs for patients and healthcare stakeholders.
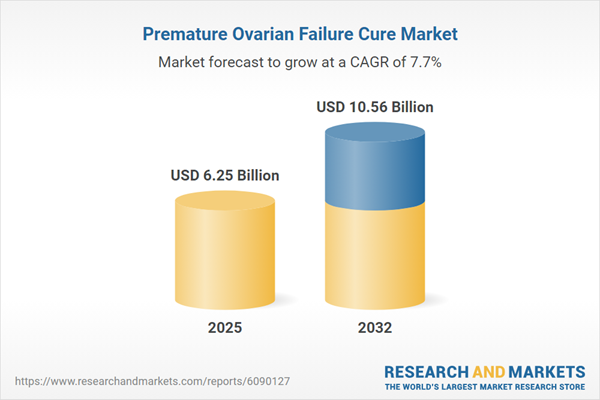
Market Snapshot: Premature Ovarian Failure Cure Market
This market has experienced notable revenue growth, supported by a robust compound annual growth rate and expanding global demand. Shifts in therapy development, such as gene and stem cell-based strategies, contribute to a dynamic competitive environment. The landscape is shaped by advances in molecular treatments, adaptive sourcing amid evolving trade policy, and active cross-sector alliances. As innovation accelerates, supply chains and investment flows increasingly focus on domestic capacities and collaborative ventures to secure market access and sustain product evolution.
Scope & Segmentation
- Treatment Type: Gene therapy, hormone replacement therapy (including combination, estrogen, and progesterone protocols), lifestyle and nutritional therapies, ovarian tissue transplantation, and stem cell therapies (adipose-derived, ovarian stem cell transplantation).
- End-User: Homecare, hospitals and clinics, research institutions.
- Distribution Channels: Hospital pharmacies, online pharmacies (brand-specific sites as well as third-party vendors), retail pharmacies.
- Regional Coverage:
- Americas: North America (United States, Canada, Mexico), Latin America (Brazil, Argentina, Chile, Colombia, Peru).
- Europe, Middle East & Africa: Europe (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland), Middle East (UAE, Saudi Arabia, Qatar, Turkey, Israel), Africa (South Africa, Nigeria, Egypt, Kenya).
- Asia-Pacific: China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan.
- Leading Companies: Bayer AG, Beam Therapeutics Inc., BioMarin Pharmaceutical Inc., Bluebird Bio, Inc., Cipla Ltd., CooperSurgical Inc., Endo International plc, Ferring Pharmaceuticals, Kitazato Corporation, Lupin Limited, Merck KGaA, Novartis AG, Orchard Therapeutics plc, Orion Corporation, OvaScience, Inc. by Millendo Therapeutics, Passage Bio, Inc., Pfizer Inc., REGENXBIO Inc., Sanofi S.A., Spark Therapeutics, Inc. by Roche Holding AG, Teva Pharmaceutical Industries Ltd., TherapeuticsMD, Inc., uniQure N.V., Vitrolife AB.
Key Takeaways
- Premature ovarian failure significantly impacts reproductive health, prompting innovation and collaboration among stakeholders from research through commercialization.
- Scientific progress in gene editing and regenerative medicine is broadening the range of restorative therapy options available, diversifying clinical pipelines across pharmaceutical and biotech enterprises.
- Hospitals, clinics, and research institutions remain fundamental for the adoption and real-world validation of emerging treatments, while telehealth-driven homecare models support patient adherence and engagement.
- Regional factors such as regulatory harmonization in Asia-Pacific, centralized approvals in Western Europe, and investment in precision medicine networks affect speed to market and access to care.
- Corporate strategies emphasize integrated innovation and distributed partnerships between pharmaceutical developers, contract manufacturers, and clinical institutions, fostering a resilient and responsive ecosystem for advanced ovarian health solutions.
Tariff Impact: 2025 United States Trade Policy
The imposition of new tariffs on imported biologics and medical materials in 2025 introduced upward pressure on manufacturing and clinical trial supply costs, driving a shift toward domestic and nearshore production networks. Collaborations between contract research organizations, biotech firms, and manufacturers have helped distribute risk and contain costs, supporting continued patient access amid supply chain adjustments. Some technology portfolios diversified through the adoption of synthetic and non-viral platforms, mitigating exposure to ongoing import constraints.
Methodology & Data Sources
This report’s findings are grounded in a multi-layered research approach, combining secondary analysis of scientific publications, regulatory updates, and financial filings with insights from in-depth interviews of clinical investigators, manufacturing specialists, and payor representatives. Scenario modeling and thematic analysis provided a comprehensive framework for evaluating technology adoption and real-world impacts within the sector.
Why This Report Matters
- Senior decision-makers gain clarity on innovation trends, regulatory strategies, and market dynamics influencing investment and product planning for premature ovarian failure therapies.
- The segmentation analysis allows for targeted identification of opportunities across end-user channels and geographies, ensuring robust planning for commercialization and partnership development.
- Actionable insights equip companies to adapt to evolving policy landscapes and optimize product pipelines to meet patient and clinical needs.
Conclusion
Driven by innovation and adaptive strategies, the premature ovarian failure cure market is set for continued advancement across research, manufacturing, and clinical adoption. This report offers the intelligence required to navigate its evolving landscape with confidence and precision.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Premature Ovarian Failure Cure market report include:- Bayer AG
- Beam Therapeutics Inc.
- BioMarin Pharmaceutical Inc.
- Bluebird Bio, Inc.
- Cipla Ltd.
- CooperSurgical Inc.
- Endo International plc
- Ferring Pharmaceuticals
- Kitazato Corporation
- Lupin Limited
- Merck KGaA
- Novartis AG
- Orchard Therapeutics plc
- Orion Corporation
- OvaScience, Inc. by Millendo Therapeutics
- Passage Bio, Inc.
- Pfizer Inc.
- REGENXBIO Inc.
- Sanofi S.A.
- Spark Therapeutics, Inc. by Roche Holding AG
- Teva Pharmaceutical Industries Ltd.
- TherapeuticsMD, Inc.
- uniQure N.V.
- Vitrolife AB
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 6.25 Billion |
| Forecasted Market Value ( USD | $ 10.56 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |