Speak directly to the analyst to clarify any post sales queries you may have.
Pioneering the Artificial Retina Frontier with Strategic Insights into Vision Restoration Technologies and Emerging Opportunities in Ophthalmic Care
The artificial retina domain represents a quantum leap in ophthalmic care, enabling patients with severe retinal degeneration to regain partial vision through sophisticated electromechanical implants. Early research experiments laid the groundwork for epiretinal stimulation, but recent advances in electrode arrays and wireless power architectures have expanded possibilities across multiple implantation sites. Today's designs leverage subretinal photodiode arrays, suprachoroidal electrode grids, and hybrid systems that integrate microelectronics directly within ocular tissues. As biomedical engineering converges with nanofabrication and materials science, these devices can deliver higher resolution, lower power consumption, and enhanced biocompatibility over chronic implantation horizons.Advancements in sensor miniaturization, hermetic packaging, and long-term biostability have accelerated clinical translation, creating a pipeline of next-generation prostheses that aim to recreate more nuanced visual patterns. At the same time, digital health integration-including remote programming and real-time performance feedback-permits personalized tuning of stimulation parameters and enables longitudinal outcome tracking.
This executive summary distills key findings from comprehensive research focused on the evolving landscape of artificial retina technologies. It begins with an overview of core drivers shaping device innovation, then traces major paradigm shifts in design and digital convergence. The impact of upcoming tariff changes on cost structures and supply chains is examined in depth, followed by segmentation, regional and competitive insights. The summary concludes with strategic recommendations designed to guide stakeholders through emerging opportunities and a transparent account of the research methodology underpinning these insights.
Unveiling the Paradigm Shifts Driving Artificial Retina Evolution from Novel Implant Pathways to Convergent Technologies Redefining Ophthalmic Restoration
Over the past decade, artificial retina technology has transitioned from single-channel prototypes to sophisticated multichannel systems capable of delivering dynamic pixel arrays directly to surviving retinal neurons. Innovations in electrode geometry, such as three-dimensional pillar arrays and flexible substrate conformations, have optimized contact with neural tissue while minimizing inflammatory responses. Concomitantly, wireless inductive coupling and ultrasonic power transfer have obviated percutaneous connectors, reducing infection risks and enhancing patient comfort. Emerging biomimetic materials, including silicon carbide coatings and soft hydrogel interfaces, have further extended implant longevity, setting the stage for fully integrated vision restoration platforms.In parallel, the integration of machine learning algorithms has enabled closed-loop vision processing, where image data from a microcamera is converted into stimulation patterns that adapt in real time to ambient light conditions and user behavior. This convergence with artificial intelligence not only amplifies perceptual fidelity but also paves the way for smart visual augmentation, supporting features like contrast enhancement and edge detection for daily living tasks.
Regulatory landscapes have simultaneously evolved to accommodate breakthrough device designations and accelerated approval pathways, particularly for therapies addressing age-related macular degeneration and retinitis pigmentosa. Reimbursement frameworks in key markets are gradually aligning with clinical evidence on quality-of-life improvements, stimulating investment in post-market outcome studies. As a result, partnerships between medical device developers, digital health innovators, and academic centers have multiplied, driving a new era of collaborative commercialization and pipeline expansion.
Assessing the Strategic Consequences of 2025 Tariff Measures on Artificial Retina Supply Chains and Cost Dynamics within the United States Ophthalmic Sector
In 2025, proposed tariff adjustments in the United States are set to impact the importation costs of specialized microelectronic components and hermetic packaging materials critical to artificial retina systems. Manufacturers sourcing high-precision sensors, custom ASICs, and biocompatible substrates from international suppliers may face incremental cost increases. These expenses could translate into higher device pricing or narrower margins unless mitigated by strategic supply-chain reengineering.As a proactive response, leading firms are evaluating alternatives such as nearshoring production lines, consolidating component sourcing with tariff-exempt partners, and leveraging bilateral trade agreements to secure duty concessions. In addition, research institutions and contract manufacturing organizations are exploring domestic fabrication of core modules to insulate long-term development budgets from tariff volatility. Despite potential cost headwinds, these adjustments may catalyze innovation in local manufacturing capabilities, driving investments that ultimately enhance resilience and reduce dependency on single-region supply sources.
Deconstructing the Multifaceted Artificial Retina Market through Implantable Device Typologies Indications and End Users Reveal Strategic Investment Vectors
Device architecture segmentation reveals three principal approaches to implantation. Epiretinal implants position electrode arrays on the retinal surface to stimulate ganglion cells directly, while subretinal devices nestle photodiodes beneath the degenerated photoreceptor layer to capture light and transduce signals. Suprachoroidal implants, in contrast, occupy the potential space between the choroid and sclera, offering less invasive surgery and reduced tissue disruption. Each implantation strategy demands unique surgical techniques, engineering trade-offs, and rehabilitation protocols, which in turn shape clinical adoption and long-term performance outcomes.Clinical indication segmentation centers on two predominant retinal diseases. Age-related macular degeneration accounts for a substantial portion of vision-loss burdens in aging populations, driving demand for central vision restoration. Retinitis pigmentosa, a genetically heterogeneous group of disorders, often presents in younger cohorts and necessitates earlier intervention strategies that balance device longevity with adaptive stimulation schemes for peripheral and night vision functions.
End user segmentation encompasses diverse channels of delivery and research. Hospitals and eye care clinics serve as primary implantation sites, integrating surgical workflows with post-operative visual rehabilitation services. Ophthalmologists and retinal specialists in private practice frequently lead patient screening, pre-surgical counseling, and individualized programming sessions. Meanwhile, research institutions and academic centers conduct pivotal trials, refine stimulation protocols, and spearhead novel device concepts in collaboration with industry partners.
Examining Regional Divergence in Artificial Retina Adoption across Americas Europe Middle East Africa and Asia Pacific through Healthcare Systems and Policy
In the Americas, robust reimbursement frameworks and established clinical trial networks have accelerated adoption of advanced artificial retina systems. Leading healthcare institutions in North America provide access to interdisciplinary vision centers that combine specialized surgical expertise with rehabilitative support services. Latin American markets, while less mature, exhibit growing interest in collaborative research initiatives and technology transfer programs, often sponsored by regional consortia focused on public health challenges related to an aging demographic.Europe, the Middle East, and Africa present a heterogeneous landscape shaped by divergent regulatory harmonization efforts and infrastructure capacities. Western European countries benefit from coordinated approval pathways and centralized funding schemes that facilitate multicenter studies. Conversely, emerging markets in the Gulf region and sub-Saharan Africa grapple with limited specialist availability and distribution challenges, yet strategic public-private partnerships are beginning to address gaps in access and training.
Asia-Pacific is characterized by dynamic growth prospects fueled by sizable patient populations and ambitious government initiatives in biotech innovation. Markets such as Japan and South Korea possess advanced manufacturing ecosystems for semiconductor lasers and bioelectronics, enabling domestic device development. India and Southeast Asia are rapidly expanding retinal screening programs and forging alliances that integrate tele-ophthalmology with implantable prosthesis deployment, positioning the region as a future hub for both clinical adoption and manufacturing scale-up.
Profiling Leading Innovators in Artificial Retina Technology and Strategic Collaborations Fueling Competitive Dynamics and Next-Generation Partnerships
Leading innovators in the artificial retina space have formed strategic alliances with semiconductor manufacturers to co-develop custom ASICs and microLED arrays that drive pixel density enhancements. Cross-industry partnerships between optoelectronics firms and ophthalmic device specialists are yielding novel sensor modules that boost signal fidelity while minimizing footprint. Meanwhile, collaboration with materials science experts has produced next-generation electrode coatings that mitigate fibrotic encapsulation and extend functional electrode lifespan.Start-ups focusing on neuromodulation are leveraging venture capital to advance clinical-stage pipelines, often in close collaboration with academic vision science centers. These entities bring agility to iterative device testing, channeling real-world performance data into rapid prototyping cycles. At the same time, established medical device conglomerates are investing in in-house research labs and acquisition of smaller innovators to broaden their portfolio across epiretinal, subretinal, and suprachoroidal modalities.
As a result, competitive dynamics center on speed to market, intellectual property breadth, and the ability to integrate digital health platforms that support remote programming and outcomes monitoring. Companies that can demonstrate interoperability with rehabilitation software, patient-friendly user interfaces, and robust training programs for surgeons and vision therapists are poised to capture premium positioning in this nascent yet rapidly evolving industry.
Delivering Practical Strategic Recommendations to Drive Commercialization Acceleration and Collaborative Pipeline Optimization in the Artificial Retina Ecosystem
To capitalize on emerging opportunities in this transformative sector, industry leaders should prioritize the integration of artificial intelligence into stimulation algorithms to improve perceptual quality and broaden use case scenarios. By investing in adaptive software platforms that learn from individual patient responses, developers can differentiate offerings and deepen clinical value propositions.Strengthening regional manufacturing footprints through joint ventures or strategic investments will not only mitigate supply-chain disruptions but also position firms to access localized reimbursement schemes. Establishing co-development agreements with contract manufacturers in tariff-sensitive geographies can unlock cost advantages while preserving design control.
Cultivating multidisciplinary consortia that bring together surgeons, vision scientists, rehabilitation specialists, and patient advocacy groups will accelerate clinical adoption and generate real-world evidence. Such collaborations can refine post-implantation care pathways, inform outcome metrics, and support reimbursement negotiations.
Finally, embedding robust cybersecurity protocols and data privacy safeguards in remote programming solutions will build trust with regulatory bodies and end users, ensuring that next-generation artificial retina platforms meet the highest standards for patient safety and data integrity.
Outlining Comprehensive Research Methods Combining Primary Expert Engagement with Mixed Quantitative and Qualitative Validation for Artificial Retina Insights
This research employed a comprehensive approach combining industry expert interviews, secondary literature analysis, and data triangulation to ensure robust insights. Primary consultations were conducted with leading ophthalmic surgeons, biomedical engineers, and policy experts across North America, Europe, and Asia Pacific to capture diverse perspectives on device performance, regulatory challenges, and market dynamics.Secondary research encompassed peer-reviewed journals, clinical trial registries, patent filings, and publicly available regulatory documents. Quantitative data from manufacturer reports were cross-referenced with clinical outcome studies to validate performance benchmarks. A structured validation process reconciled discrepancies through follow-up interviews and iterative review cycles, delivering a transparent and reproducible methodological framework.
Synthesizing Critical Insights on the Evolution Trajectory and Strategic Imperatives Shaping Future Developments in the Artificial Retina Domain
As the artificial retina field advances toward higher resolution, safer implantation techniques, and digital health integration, stakeholders must remain attuned to shifts in supply-chain economics and reimbursement policies. The interplay between neuromodulation technology and machine learning will be a critical determinant of device efficacy and patient satisfaction, unlocking new applications beyond central vision restoration.Ultimately, success in this domain will hinge on fostering collaborative ecosystems that bring together medical device manufacturers, academic research institutions, clinical practitioners, and patient communities. By aligning technical innovation with patient-centric care models and adaptive policy frameworks, the industry can realize the full potential of artificial retina systems to transform lives.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Implantable Retinal Devices
- Epiretinal Implants
- Subretinal Implants
- Suprachoroidal Implants
- Indication
- Age-Related Macular Degeneration (AMD)
- Retinitis Pigmentosa (RP)
- End User
- Hospitals & Eye Care Clinics
- Ophthalmologists & Retinal Specialists
- Research Institutions & Academic Centers
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Aetna Inc.
- Altris, Inc.
- Axorus SAS
- Bausch + Lomb Corporation.
- Bionic Vision Technologies
- BrightFocus Foundation
- iBIONICS
- Kurzweil Network
- Labtician Ophthalmics, Inc.
- LambdaVision, Inc.
- Optobionics Incorporated.
- Orient Europharma Co., Ltd
- Pixium Vision by Science Corporation
- Vivani Medical, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Artificial Retina market report include:- Aetna Inc.
- Altris, Inc.
- Axorus SAS
- Bausch + Lomb Corporation.
- Bionic Vision Technologies
- BrightFocus Foundation
- iBIONICS
- Kurzweil Network
- Labtician Ophthalmics, Inc.
- LambdaVision, Inc.
- Optobionics Incorporated.
- Orient Europharma Co., Ltd
- Pixium Vision by Science Corporation
- Vivani Medical, Inc.
Table Information
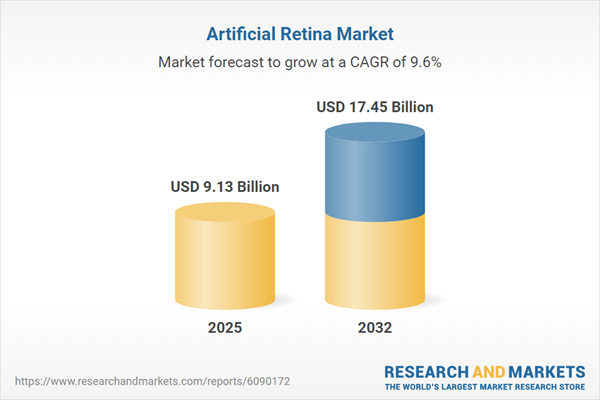
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 9.13 Billion |
| Forecasted Market Value ( USD | $ 17.45 Billion |
| Compound Annual Growth Rate | 9.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |