Speak directly to the analyst to clarify any post sales queries you may have.
The personalized medicine software market is undergoing rapid transformation, driven by advances in data integration, analytics, and cross-sector collaboration. Senior leaders need a clear overview of core growth drivers, strategic opportunities, and potential disruptions shaping this sector.
Market Snapshot: Personalized Medicine Software Market Size and Outlook
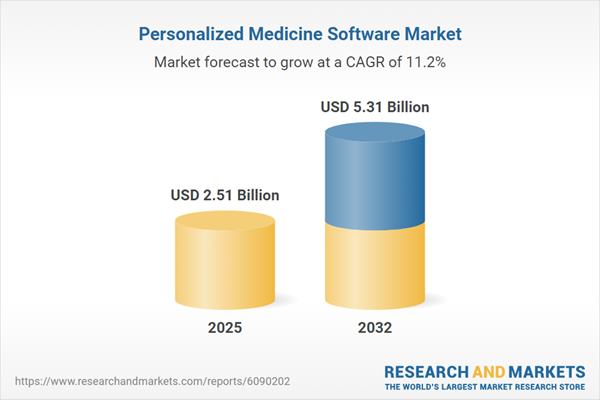
The personalized medicine software market grew from USD 2.27 billion in 2024 to USD 2.51 billion in 2025. It is expected to continue at a CAGR of 11.19%, reaching USD 5.31 billion by 2032. The sector is propelled by increasing demand for scalable, patient-centric digital platforms, rising complexity in healthcare data, and advances in precision diagnostics and analytics. As the landscape matures, innovative vendors and healthcare facilities are adopting software to streamline clinical decision-making, enhance outcomes, and support robust research initiatives.
Scope & Segmentation: In-Depth Coverage of Functions, Applications, Deployment, and Regions
- Type: EHR integration, genomic data analysis, patient satisfaction and risk assessment modules, predictive analysis, and treatment recommendation engines.
- Application: Cardiology, neurology, oncology.
- Deployment Mode: Cloud-based and on-premises solutions.
- End User: Healthcare providers (clinics, hospitals), research institutes (academic institutes, independent labs).
- Regions: Americas (United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru), Europe, Middle East & Africa (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland, United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel, South Africa, Nigeria, Egypt, Kenya), Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan).
- Key Players: Includes companies such as 23andMe, 3D Systems, Caris Life Sciences, Epic Systems, Flatiron Health, Foundation Medicine, Genelex, Genomind, Health Fidelity, IBM, Illumina, Innowise, Invitae, NantHealth, OSP Labs, Personal Genome Diagnostics, Roche Holding, Strand Life Sciences, Syapse, Tempus AI, and others.
Key Takeaways: Strategic Insights for Decision-Makers
- Emerging opportunities lie at the intersection of AI-powered analytics and clinical workflow integration, enabling tailored diagnostics and improved patient pathways.
- Vendor collaboration with hardware manufacturers and cross-border partners helps organizations adapt to interoperability challenges and regulatory demands.
- Patient engagement tools and holistic management modules are becoming essential for both adherence and outcomes improvement.
- Modular and scalable platforms are favored to address evolving deployment needs and varying levels of data sovereignty.
- Regional expansion is driven by differing regulatory environments and investment in R&D, fostering targeted innovation and customization.
Tariff Impact: Navigating New Cost Structures and Supply Strategies
The introduction of United States tariffs in 2025 has significantly influenced procurement and deployment choices. Software firms reliant on imported hardware have experienced higher costs, leading to renewed focus on domestic manufacturing and bundled licensing strategies. Shifting trade regulations are also reshaping cross-border agreements and cloud deployment preferences, prompting vendors and end users to explore regional infrastructure and flexible service frameworks that control costs while maintaining continuity of care.
Personalized Medicine Software: Research Methodology & Data Sources
This report leverages a multimodal methodology, combining primary interviews with clinicians, executives, and technical experts, comprehensive literature reviews, patent and technology adoption data, and expert panel validation. Scenario modeling and iterative segmentation analysis support robust, objective insights about current trends and future projections.
Why This Report Matters for Senior Leaders
- Provides actionable intelligence for aligning software investments with regulatory, technological, and regional shifts in the personalized medicine ecosystem.
- Identifies how emerging deployment models, innovative collaborations, and evolving care pathways will shape near- and long-term growth and operational resilience.
Conclusion
This report presents a comprehensive and strategic review of the personalized medicine software market. By examining market dynamics, disruptive forces, and actionable trends, it equips leaders to make informed, future-ready decisions in precision healthcare.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Personalized Medicine Software market report include:- 23andMe, Inc.
- 3D Systems, Inc.
- Caris Life Sciences, Inc.
- Epic Systems Corporation
- Flatiron Health, Inc.
- Foundation Medicine, Inc.
- Genelex Corporation
- Genomind, Inc.
- Health Fidelity, Inc.
- IBM Corporation
- Illumina, Inc.
- Innowise
- Invitae Corporation
- NantHealth, Inc.
- OSP Labs
- Personal Genome Diagnostics, Inc.
- Roche Holding AG
- Strand Life Sciences Pvt. Ltd.
- Syapse, Inc.
- Tempus AI, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 2.51 Billion |
| Forecasted Market Value ( USD | $ 5.31 Billion |
| Compound Annual Growth Rate | 11.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |