Speak directly to the analyst to clarify any post sales queries you may have.
The Access & Dissection Devices Market is evolving as healthcare providers seek innovative solutions for enhanced surgical outcomes, increased procedural efficiency, and streamlined integration of minimally invasive techniques across specialties. As technology transforms clinical practice, understanding the market’s distinct drivers and strategic opportunities is critical for senior decision-makers.
Market Snapshot: Access & Dissection Devices Market Overview
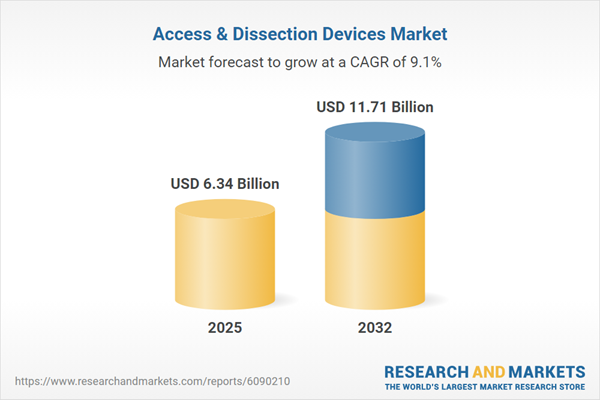
The Access & Dissection Devices Market grew from USD 5.84 billion in 2024 to USD 6.34 billion in 2025, signaling sustained momentum. With an expected CAGR of 9.08%, the market is poised to reach USD 11.71 billion by 2032, reflecting strong demand for devices supporting minimally invasive procedures and advanced surgical environments.
Scope & Segmentation: Comprehensive Analysis of Market Dimensions
- Product Categories: Access devices (cannulas, laparoscopic instruments, trocars), and dissection devices (electrosurgical, laser, mechanical, ultrasound surgical devices).
- Surgery Types: Minimally invasive surgery and open surgery.
- Clinical Applications: Cardiac surgery, general surgery, neurosurgery, orthopedic surgery, vascular surgery.
- End Users: Hospitals, ambulatory surgical centers, specialty clinics.
- Regional Coverage: Americas (including North America, Latin America), Europe, Middle East & Africa, Asia-Pacific.
- Featured Companies: B. Braun AG, Medtronic PLC, 3M Company, Applied Medical Resources Corporation, Becton, Dickinson and Company, Boston Scientific Corporation, CONMED Corporation, Cook Group Incorporated, Friedrich Daniels GmbH, GE Healthcare Technologies, Inc., GPC Medical Ltd., Herniamesh S.r.l., Integra LifeSciences Holdings Corporation, Johnson & Johnson Services, Inc., Olympus Corporation, Omega Surgical Instruments Inc., P.W. Coole & Son Limited, Paragon Medical, Inc., Peters Surgical Private Limited, Scanlan International Inc., Stryker Corporation, W.L. Gore & Associates.
Key Takeaways for Senior Decision-Makers
- Device design innovation is delivering increased precision and efficiency, directly benefiting surgeons aiming to reduce incision size and optimize recovery timelines.
- Technological advancements, such as real-time sensing and multi-energy platforms, are minimizing collateral tissue impact and shortening procedure durations.
- Integration of ergonomic features and digital imaging is influencing clinician preferences and supporting workflow improvements in complex surgeries.
- Rising adoption of minimally invasive surgery has redefined training protocols and procedural strategies, demanding adaptable device sets for varied clinical scenarios.
- Strategic partnerships, including collaborations with robotics and digital health firms, are strengthening market positioning by enabling holistic procedural solutions and service enhancements.
- Regional market differences in reimbursement, regulatory frameworks, and healthcare infrastructure require localized approaches to ensure effective adoption and expansion.
Tariff Impact: Navigating Supply Chain Pressures
Recent United States tariffs have created upward pressure on sourcing costs in the access and dissection devices market. Stakeholders have responded by optimizing production footprints, exploring nearshoring, and qualifying new suppliers to mitigate supply volatility and protect profitability. Organizations with robust, vertically integrated supply chains are better positioned to manage disruptions and maintain competitive pricing strategies.
Methodology & Data Sources
This report is based on rigorous primary and secondary research. Data collection included interviews with surgeons, procurement experts, and device engineers, complemented by discussions with manufacturing and distribution leaders. Regulatory filings, peer-reviewed publications, and industry white papers were reviewed, and data triangulation ensured consistency across all findings.
Why This Report Matters
- Equips leaders with actionable insights to align R&D, supply chain, and market entry strategies with evolving clinician needs and regulatory landscapes.
- Provides a clear understanding of product, clinical, and geographic segments, enabling targeted portfolio management and risk mitigation planning.
- Supports strategic decisions by mapping technology adoption trends, partnership opportunities, and investment priorities in the competitive landscape.
Conclusion
The Access & Dissection Devices Market is being reshaped by integrated technologies, adaptive regulatory pathways, and evolving surgical requirements. Leaders who leverage these insights can optimize their device portfolios and strengthen their organizations’ competitive positions in a dynamic industry landscape.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Access & Dissection Devices Market report include:- B. Braun AG
- Medtronic PLC
- 3M Company
- Applied Medical Resources Corporation
- Becton, Dickinson, and Company
- Boston Scientific Corporation
- CONMED Corporation
- Cook Group Incorporated
- Friedrich Daniels GmbH
- GE Healthcare Technologies, Inc.
- GPC Medical Ltd.
- Herniamesh S.r.l.
- Integra LifeSciences Holdings Corporation
- Johnson & Johnson Services, Inc.
- Olympus Corporation
- Omega Surgical Instruments Inc.
- P.W. Coole & Son Limited
- Paragon Medical,Inc.
- Peters Surgical Private Limited
- Scanlan International Inc.
- Stryker Corporation
- W.L. Gore & Associates
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 6.34 Billion |
| Forecasted Market Value ( USD | $ 11.71 Billion |
| Compound Annual Growth Rate | 9.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |