Speak directly to the analyst to clarify any post sales queries you may have.
Setting the Stage for Understanding the Evolving Landscape of Experimental Cynomolgus Monkey Utilization in Preclinical Research Applications Worldwide
The landscape of preclinical research has witnessed a profound evolution driven by the unparalleled relevance of the cynomolgus monkey model. As pharmaceutical and biotechnology organizations pursue novel therapeutics, the predictive validity offered by these nonhuman primates has become indispensable. Their physiological and immunological proximity to humans enables a deeper understanding of drug efficacy, safety, and immunogenicity, laying the foundation for faster and more reliable clinical translation.Against this backdrop, stakeholders across academia, contract research organizations, and industry are recalibrating their strategies to harness the full potential of cynomolgus monkey studies. Increased investment in colony management, enhanced welfare protocols, and advanced analytical platforms underscore a commitment to scientific rigor and ethical stewardship. Consequently, research teams can generate higher fidelity data while adhering to the most stringent regulatory requirements.
This executive summary synthesizes the critical factors reshaping this market. Starting with an exploration of transformative shifts, it examines the impact of recent tariff policies, delivers nuanced segmentation insights, maps regional dynamics, profiles leading organizations, offers actionable recommendations, and outlines the rigorous mixed method research approach employed. The conclusion distills strategic imperatives, setting the stage for informed decision-making and future innovation.
Exploring the Crucial Transformations Reshaping Experimental Cynomolgus Monkey Research Through Technological and Ethical Advances
Pioneering technological innovations and evolving ethical frameworks are redefining the contours of experimental cynomolgus monkey research. Gene editing tools such as CRISPR have unlocked new possibilities for creating precise disease models, enabling researchers to probe genetic disorders with unprecedented specificity. Simultaneously, high-resolution imaging modalities and telemetry systems are generating real-time physiological data, elevating both the depth and breadth of safety and efficacy assessments.Concurrently, regulatory bodies across major markets are strengthening guidelines to ensure humane treatment and welfare monitoring, reinforcing the principles of reduction, refinement, and replacement. As organizations adopt advanced enrichment strategies and noninvasive monitoring techniques, the harmonization between scientific objectives and ethical obligations has never been stronger. This confluence of innovation and responsibility is fostering transparency, trust, and reproducibility in preclinical research.
Moreover, supply chain dynamics have undergone seismic shifts. Strategic partnerships with specialized breeding centers, diversification of sourcing regions, and the adoption of digital traceability systems are mitigating risks associated with global distribution. Collectively, these transformations are cultivating a resilient and agile ecosystem, prepared to address the rising demand for robust translational data.
Analyzing How Recent United States Tariffs Have Redefined Supply Chain Dynamics and Cost Structures for Cynomolgus Monkey Studies in Twenty Twenty Five
The introduction of heightened import duties by the United States in twenty twenty five has precipitated a significant recalibration of supply chain strategies for organizations relying on cynomolgus monkeys. As tariffs drove up the cost of procurement, research budgets faced unprecedented pressure, compelling teams to reevaluate sourcing arrangements and seek alternative geographic partners. This shift has catalyzed a broader realignment, with many institutions exploring breeding facilities in regions offering more favorable trade terms.Over time, procurement officers and research directors have renegotiated long-term agreements to include cost-sharing mechanisms and volume discounts, thereby insulating critical programs from sudden price escalations. At the same time, firms have strengthened relationships with regional breeding centers in Asia-Pacific and parts of Europe, Middle East & Africa, fostering collaborative frameworks that balance cost efficiencies with rigorous quality standards. These adaptations not only preserve project timelines but also reinforce supply chain resilience against future policy fluctuations.
Consequently, project teams have implemented comprehensive risk assessment protocols that integrate tariff scenarios into financial planning. By embedding these considerations early in the program lifecycle, stakeholders are better equipped to navigate regulatory shifts and maintain continuity in preclinical pipelines.
Uncovering Deep Insights Into Experimental Cynomolgus Monkey Research Through Nuanced Segmentation Across Multiple Critical Dimensions of Study Design
A multifaceted view of the cynomolgus monkey research market emerges when data are segmented across critical dimensions. Based on research type, drug efficacy testing stands at the forefront, driven by burgeoning interest in biologics and cell therapies, while small molecule evaluations continue to sustain a steady demand. Pharmacology and toxicology studies, spanning acute toxicity, carcinogenicity assessments, and chronic toxicity investigations, form a vital pillar, underpinning the safety profiles of emerging therapeutics. Parallel focus on immunogenicity studies and preclinical safety evaluation ensures that vaccine candidates and other novel modalities meet rigorous risk-benefit thresholds.When viewed through the prism of health status, disease models are increasingly tailored to replicating specific pathophysiological conditions, whereas genetic variants unlock deeper insights into pharmacogenomic responses. The inclusion of healthy cynomolgus monkeys remains essential for establishing baseline controls and enhancing comparative analyses. Gender segmentation further refines study design, as researchers explore sex-specific immunological responses, endocrine effects, and metabolic variations between female and male subjects.
End-user perspectives reveal that academic and research institutes drive foundational science, while contract research organizations scale complex preclinical services for multinational clients. Pharmaceutical and biotechnology companies leverage these insights to accelerate translational workflows and optimize go-to-clinic strategies. Finally, disease area segmentation highlights a diverse spectrum of applications, from cardiovascular investigations and genetic/genomic research to immunology, infectious diseases, neurological studies, oncology endeavors, and respiratory disease modeling. Together, these overlapping dimensions paint a rich tapestry of demand drivers and strategic opportunities.
Revealing How Regional Dynamics Shape the Experimental Cynomolgus Monkey Landscape Across the Americas Europe Middle East Africa and Asia Pacific
Regional dynamics exert a powerful influence on the availability, cost, and regulatory compliance of cynomolgus monkey studies. In the Americas, state-of-the-art breeding facilities and an expansive network of contract research organizations support some of the most sophisticated preclinical pipelines. However, stringent regulatory oversight and higher operating expenses compel organizations to balance scientific ambitions with budgetary constraints.In Europe, Middle East & Africa, harmonization initiatives led by central agencies are streamlining approval processes, fostering a more predictable environment for multi-country studies. Emerging markets in the Middle East and select African nations are also investing in local breeding and research infrastructure, thereby reducing reliance on long-distance imports and enhancing regional self-sufficiency.
Across Asia-Pacific, cost-effective colony management, coupled with government-backed research grants, has propelled rapid expansion of domestic pharmaceutical research. Leading markets are establishing centers of excellence to meet escalating demand, while smaller economies are leveraging strategic partnerships to access advanced capabilities. As a result, this region has become a pivotal node in the global ecosystem, balancing affordability with ever-improving standards of animal welfare and data quality.
Highlighting Leading Organizations Driving Innovation Strategic Collaborations and Quality Enhancements in the Experimental Cynomolgus Monkey Research Ecosystem
The competitive landscape is marked by organizations that excel in breeding, regulatory support, and integrated research services. Breeding centers investing in genetic characterization and health monitoring protocols have set new benchmarks for colony quality. Collaboration between these centers and technology providers offering real-time welfare monitoring has further elevated standards, ensuring both ethical compliance and data integrity.Contract research organizations have expanded their service portfolios to include specialized toxicology, immunogenicity, and advanced imaging solutions. By forging strategic alliances with biotechnology innovators, these firms deliver end-to-end preclinical workflows, seamlessly integrating study design, execution, and data interpretation. Pharmaceutical companies, in turn, are forging partnerships with academic institutions to accelerate translational research while maintaining access to cutting-edge facilities.
This landscape of interdependent players underscores the importance of cross-sector collaboration. Organizations that combine breeding excellence with regulatory expertise and technological innovation are best positioned to capture new growth opportunities and to support the next generation of therapeutic breakthroughs.
Delivering Practical Strategic Recommendations to Optimize Preclinical Research Programs Utilizing Experimental Cynomolgus Monkeys for Enhanced Study Outcomes
To thrive in this complex environment, industry leaders should diversify sourcing strategies by establishing partnerships with multiple breeding centers across regions. Such an approach mitigates exposure to trade policy fluctuations and strengthens supply chain resilience. Concurrently, investing in digital monitoring platforms for animal welfare and data collection can enhance transparency, reduce error margins, and accelerate insights generation.Engaging proactively with regulatory agencies is equally crucial. Early alignment on study protocols and welfare standards can shorten approval timelines and reduce the risk of costly delays. Moreover, integrating multidisciplinary project teams that combine pharmacologists, geneticists, and data scientists fosters innovative study designs and comprehensive data interpretation. Standardizing data management frameworks across departments can further streamline workflows and facilitate cross-functional learning.
Finally, cultivating collaborative networks with academic institutions and technology partners enables access to specialized expertise, novel methodologies, and emerging biomarkers. By embedding these strategic initiatives within organizational roadmaps, stakeholders can optimize resource allocation, accelerate time to clinic, and maintain the highest standards of scientific and ethical rigor.
Explaining the Rigorous Mixed Method Research Methodology Employed to Develop an Overview of Experimental Cynomolgus Monkey Utilization in Preclinical Research
The development of this market analysis was guided by a mixed method research design, ensuring robustness through the convergence of qualitative and quantitative insights. Primary research included in-depth interviews with key opinion leaders across academia, contract research organizations, and pharmaceutical firms, providing firsthand perspectives on evolving requirements and emerging challenges. Field visits to breeding and research facilities enriched the contextual understanding of operational practices and welfare protocols.Secondary research encompassed the review of peer-reviewed publications, regulatory guidelines, and proprietary databases to capture historical trends and benchmark standards. Data triangulation techniques were applied to reconcile disparate sources, enhancing the credibility of findings. Segmentation frameworks-covering research type, health status, gender, end-user, and disease area-were systematically validated through industry consultations.
Finally, strategic validation workshops were convened with senior stakeholders to refine insights and ensure alignment with market realities. The result is a comprehensive, evidence-based overview that empowers decision-makers to navigate the dynamic terrain of experimental cynomolgus monkey utilization.
Concluding Observations on the Future Trajectory and Strategic Imperatives Shaping the Experimental Cynomolgus Monkey Research Ecosystem through a Global Lens
In conclusion, the experimental cynomolgus monkey market is poised at the intersection of scientific innovation, ethical responsibility, and geopolitical complexity. Technological advances in genetic editing and imaging are expanding the frontiers of translational research, while evolving welfare frameworks are reinforcing the moral contract that underpins animal-based studies. Simultaneously, tariff-induced cost pressures have spurred supply chain realignments, underscoring the need for diversified sourcing and adaptive financial planning.Segment analysis highlights the nuanced requirements of various research types, health statuses, and end-user categories, enabling stakeholders to tailor strategies for maximum impact. Regional insights reveal that while the Americas maintain leadership in advanced capabilities, Europe, Middle East & Africa and Asia-Pacific are rapidly enhancing their positions through regulatory harmonization and infrastructure investments.
As leading organizations continue to forge strategic collaborations and innovate breeding and study designs, the imperative for proactive engagement with regulatory bodies, digital transformation, and ethical stewardship has never been clearer. These strategic imperatives will shape the future trajectory of preclinical research, ensuring that experimental cynomolgus monkey studies remain a cornerstone of safe and effective therapeutic development.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Research Type
- Drug Efficacy Testing
- Biologics
- Cell Therapies
- Small Molecule Drugs
- Pharmacology & Toxicology Studies
- Acute Toxicity
- Carcinogenicity Tests
- Chronic Toxicity
- Safety Assessment
- Immunogenicity Studies
- Preclinical Safety Evaluation
- Vaccine Testing
- Drug Efficacy Testing
- Health Status
- Disease Models
- Genetic Variants
- Healthy Cynomolgus Monkeys
- Gender
- Female
- Male
- End-User
- Academic & Research Institutes
- Contract Research Organizations
- Pharmaceutical & Biotechnology Companies
- Disease Area
- Cardiovascular Studies
- Genetic / Genomic Research
- Immunology Research
- Infectious Diseases
- Neurological Research
- Oncology Research
- Respiratory Diseases
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Alpha Genesis, Inc.
- AMSBIO by Europa Biosite
- Athens Research & Technology, Inc.
- BioChain Institute Inc.
- BioChemed Services
- BioIVT LLC
- Cell Biologics, Inc.
- Creative Biolabs, Inc.
- Hainan Jingang Biotech.,Co.LTD.
- Hubei Topgene Biotechnology Co., Ltd.
- Innovative Research Inc.
- iQ Biosciences
- Lab Bioreagents
- ODIN Bioscience
- Primate Product Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Experimental Cynomolgus Monkey market report include:- Alpha Genesis, Inc.
- AMSBIO by Europa Biosite
- Athens Research & Technology, Inc.
- BioChain Institute Inc.
- BioChemed Services
- BioIVT LLC
- Cell Biologics, Inc.
- Creative Biolabs, Inc.
- Hainan Jingang Biotech.,Co.LTD.
- Hubei Topgene Biotechnology Co., Ltd.
- Innovative Research Inc.
- iQ Biosciences
- Lab Bioreagents
- ODIN Bioscience
- Primate Product Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
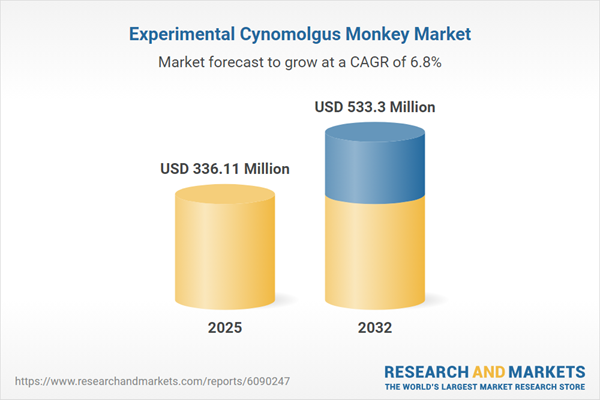
| Estimated Market Value ( USD | $ 336.11 Million |
| Forecasted Market Value ( USD | $ 533.3 Million |
| Compound Annual Growth Rate | 6.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |