Speak directly to the analyst to clarify any post sales queries you may have.
Introduction to the Critical Role of Lipid-Based Pharmaceutical Excipients in Enhancing Drug Delivery Performance and Patient Outcomes Worldwide
Lipid-based pharmaceutical excipients are foundational components that enhance drug solubility, stability, and bioavailability. These substances, derived from fatty acids, glycerides, phospholipids, alcohols, vegetable oils, and waxes, play a critical role in overcoming the challenges posed by poorly soluble active pharmaceutical ingredients. By creating specialized formulations such as nanoemulsions and self-emulsifying drug delivery systems, these excipients enable more efficient absorption and improved therapeutic outcomes. Their versatility extends across oral, parenteral, topical, nasal, and ophthalmic applications, making them indispensable in modern drug development.Over the past decade, the industry has witnessed a surge in the adoption of lipid-based excipients as pharmaceutical developers seek to deliver complex molecules with greater precision. Advances in material science have allowed formulators to fine-tune release profiles, enhance targeted delivery, and reduce adverse effects associated with high-dosage regimens. Furthermore, the shift toward personalized medicine has underscored the value of these excipients in tailoring treatments to patient-specific needs, whether adjusting ratios of short-chain to medium-chain triglycerides or leveraging specialized phospholipids to modulate membrane interactions.
Looking ahead, the landscape of lipid-based excipients is set to undergo further evolution driven by sustainability priorities, novel manufacturing technologies, and regulatory frameworks that emphasize quality and consistency. This introduction sets the stage for a deeper exploration of transformative shifts, tariff impacts, segmentation insights, regional dynamics, competitive landscapes, strategic recommendations, and the rigorous methodology underpinning this comprehensive study.
Revolutionary Transformations Shaping the Lipid-Based Pharmaceutical Excipients Landscape with Technological Advances and Regulatory Evolutions Driving Innovation
Recent years have seen transformative shifts in the lipid-based excipient domain as technological breakthroughs converge with evolving regulatory expectations. Innovations in nanoformulation methods have enabled the creation of ultra-fine emulsions and solid lipid nanoparticle platforms that dramatically improve the solubility and absorption of challenging active molecules. Simultaneously, advanced analytical techniques-such as high-resolution microscopy and spectroscopic profiling-provide deeper insights into excipient-drug interactions, facilitating more predictable performance and enhanced safety profiles.On the regulatory front, authorities are increasingly prioritizing consistency in raw material sourcing, impurity control, and process validation. This shift has galvanized manufacturers to adopt robust quality-by-design frameworks and to invest in real-time monitoring capabilities. In parallel, sustainability mandates are prompting a reevaluation of feedstock origins, encouraging the development of semi-synthetic and synthetic lipid alternatives that reduce reliance on animal or environmentally sensitive sources.
Furthermore, the rise of continuous manufacturing and digital twin modeling is reshaping production paradigms, offering scalable and cost-effective routes to high-purity excipients. These cumulative shifts underscore a broader industry transition toward agility, transparency, and sustainability, yielding a dynamic ecosystem in which innovation accelerates the development of safer, more efficacious pharmaceutical products.
Assessing the Comprehensive Effects of United States Trade Tariffs Announced for 2025 on Lipid-Based Pharmaceutical Excipient Supply Chains and Cost Structures
The introduction of United States tariffs in 2025 has imposed new pressures on the global supply chain for lipid-based excipients. Tariffs on key raw materials-ranging from hydrogenated phosphatidylcholine to coconut oil derivatives-have elevated procurement costs and triggered cascading effects across formulation budgets. In response, manufacturers are exploring alternative supply routes, including increased domestic sourcing and regional partnerships, to mitigate the financial impact.As cost structures shift, excipient producers and pharmaceutical companies alike are reassessing their supplier portfolios and inventory strategies. Collaborative agreements between ingredient suppliers and CDMOs are becoming more prevalent, fostering shared risk models and just-in-time delivery frameworks that maintain access to critical feedstocks. Meanwhile, the search for tariff-exempt or lower-duty origin points is driving an uptick in production initiatives across Asia-Pacific locales with favorable trade arrangements.
Despite these challenges, the drive for high-performance drug formulations remains steadfast. Industry leaders are leveraging process efficiencies and formulation optimization to absorb tariff-related cost increases without compromising product efficacy. In doing so, they reinforce the strategic imperative of supply chain resilience and underscore the adaptability required to navigate evolving trade policies.
Key Segmentation Perspectives Revealing Diverse Product Types Sources Applications Functionalities and End-User Demands in Lipid-Based Pharmaceutical Excipients
Understanding the multifaceted nature of lipid-based excipients requires a detailed view of product typologies, raw material sources, formulation applications, functional roles, and end-user profiles. In terms of product types, developers engage with a range of fatty acids, fatty alcohols such as cetyl alcohol and stearyl alcohol, glycerides including mixed glycerides, monoglycerides like glycerol monostearate and monolaurin, and triglycerides spanning long chain, medium chain, and short chain variants. Formulators also rely on phospholipids such as hydrogenated phosphatidylcholine and soybean lecithin, complemented by vegetable oils like coconut and sesame oil, as well as waxes drawn from beeswax and carnauba wax.Raw material sourcing further distinguishes excipient categories, with natural lipids derived directly from plant or animal origins, semi-synthetic derivatives offering tailored molecular properties, and fully synthetic lipids engineered for maximum consistency. Each source class brings unique attributes to formulation design and quality control regimes.
Applications span nasal sprays, ophthalmic preparations, oral drug delivery systems in capsule and tablet forms, parenteral solutions including intravenous lipid emulsions and vaccine adjuvants, and topical formulations in creams and patches. Within these matrices, excipients serve distinct functionalities, acting as binders and fillers, emulsifiers and co-emulsifiers, lubricants, penetration enhancers, solubilizing agents, and sustained or controlled release mediators.
Finally, academic and research institutes fuel discovery and early-stage validation, while contract development and manufacturing organizations accelerate scale-up. Contract research organizations provide critical testing and regulatory support, and pharmaceutical and biopharmaceutical manufacturers drive commercial adoption, underscoring the interconnected end-user ecosystem that shapes excipient innovation.
Regional Dynamics and Opportunities in the Americas Europe Middle East Africa and Asia-Pacific Across Lipid-Based Pharmaceutical Excipients Deployment
Regional dynamics exert a profound influence on both the supply and demand sides of lipid-based excipients. In the Americas, advanced pharmaceutical markets in the United States and Canada emphasize high-purity excipients for complex parenteral formulations and specialty oral therapies. The presence of world-class research institutions and a strong CDMO network further stimulates innovation and fosters nimble responses to trade policy changes.In Europe, the Middle East, and Africa, stringent regulatory frameworks and sustainability commitments steer the adoption of responsibly sourced lipids and green manufacturing practices. Leading pharmaceutical hubs in Western Europe collaborate closely with academic centers to pioneer novel emulsification technologies, while emerging markets in Eastern Europe and the Gulf region expand capacity to serve both local and export demands.
Asia-Pacific stands out as a powerhouse for both raw material production and cost-effective manufacturing. Countries with abundant coconut and sesame oil resources, combined with evolving synthetic lipid capabilities, support a robust supply ecosystem. Rapid growth in markets such as India, China, and Southeast Asia fuels demand for generic oral formulations, injectables, and vaccine adjuvants, driving CDMO expansion and incentivizing innovation in scalable lipid-based systems.
These regional insights illustrate how geographic factors, regulatory landscapes, and resource endowments intersect to shape the global excipient framework, challenging stakeholders to tailor strategies to each market's unique characteristics.
Competitive Profiles and Strategic Movements of Leading Corporations Driving Innovation Production and Partnership Strategies in the Lipid-Based Pharma Excipient Sector
Within the competitive landscape, several industry participants stand out for their strategic positioning and innovation prowess. Leading specialty chemical companies have expanded their portfolios through targeted acquisitions of niche lipid producers, integrating novel phospholipid technologies and advanced triglyceride fractions. At the same time, CDMOs are forging deep partnerships with excipient suppliers to develop co-branded formulations and streamline transfer to commercial production.R&D-focused enterprises are differentiating through proprietary emulsification platforms and intellectual property filings that protect next-generation self-emulsifying and nanoemulsion systems. Collaborative consortia involving academic laboratories and industrial partners are accelerating early-stage feasibility studies, often funded through government or private grants aimed at addressing solubility and delivery challenges.
Moreover, manufacturers with vertically integrated supply chains are capitalizing on traceability and quality control advantages, offering comprehensive documentation packages and real-time monitoring services. These firms frequently secure long-term agreements with pharmaceutical customers, locking in supply and ensuring consistent performance of high-value excipients.
Overall, the competitive landscape reflects a balance between large, diversified chemical players pursuing scale and emerging specialists driving innovation through agile development models and close customer collaboration.
Strategic Recommendations Empowering Industry Leaders to Leverage Technological Trends Regulatory Changes and Market Dynamics in Lipid-Based Pharmaceutical Excipients
To navigate the rapidly evolving landscape, industry leaders should prioritize supply chain diversification by establishing relationships with multiple feedstock providers and regional manufacturing partners. Aligning procurement strategies with transparent traceability initiatives will not only mitigate tariff and geopolitical risks but also support sustainability commitments.In parallel, investment in green chemistry and bio-based synthetic lipid platforms will position organizations at the forefront of regulatory and environmental standards. Embracing continuous manufacturing, real-time analytics, and digital process control can further enhance product consistency and reduce time to market.
Organizations must also cultivate cross-sector collaborations, engaging with academic research networks and clinical stakeholders to co-develop tailored excipient solutions for emerging modalities such as biologics, gene therapies, and personalized nutraceuticals. Strengthening partnerships with CDMOs and contract research organizations will enable flexible scale-up and regulatory support.
Finally, proactive regulatory engagement-through participation in working groups, publishing white papers, and contributing to industry standards-will ensure that excipient specifications and guidelines evolve in alignment with innovation trajectories. By adopting these strategic measures, companies can secure a competitive edge, unlock new application spaces, and sustain growth in the global excipient marketplace.
Rigorous Research Methodology Incorporating Primary Data Collection Secondary Literature Review and Advanced Analytical Frameworks for Market Insights
This study employs a rigorous mixed-methods approach to deliver robust insights into lipid-based excipients. Primary research activities included in-depth interviews with senior executives from pharmaceutical manufacturers, CDMOs, contract research organizations, and academic institutions. These dialogues provided firsthand perspectives on supply chain challenges, formulation innovations, and regulatory expectations.In parallel, secondary research encompassed an extensive review of peer-reviewed journals, technical conference proceedings, patent databases, and regulatory agency publications. Data points were cross-validated through triangulation, ensuring accuracy and consistency. Key process parameters, material specifications, and adoption barriers were mapped against industry best practices.
Quantitative analysis integrated statistical modeling to examine historical trends in trade flows, raw material procurement costs, and excipient portfolio expansions. Qualitative findings were synthesized through thematic coding, enabling the identification of emerging patterns in technology adoption and market entry strategies.
Finally, the study's conclusions and recommendations were vetted through validation workshops with industry thought leaders. This iterative process ensured that the final deliverables reflect both empirical evidence and strategic foresight, equipping stakeholders with actionable intelligence for informed decision-making.
Concluding Perspectives Highlighting Core Findings Implications and Future Directions for Lipid-Based Pharmaceutical Excipients in Drug Delivery Solutions
This executive summary has highlighted the paramount importance of lipid-based excipients in modern pharmaceutical formulation, underscored by their ability to enhance solubility, stability, and targeted delivery. Transformative shifts in nanoformulation technologies, regulatory oversight, and sustainability imperatives are reshaping production and development paradigms, while trade policy changes demand agile supply chain strategies.Through a granular segmentation lens, the diversity of product types, raw material sources, application areas, functional roles, and end-user ecosystems becomes clear. Regional insights reveal distinct market drivers and growth levers across the Americas, Europe Middle East Africa, and Asia-Pacific. Meanwhile, competitive analysis underscores the strategic maneuvers of chemical innovators, specialty producers, and CDMOs driving the sector forward.
In synthesizing these findings, the importance of proactive engagement with emerging trends, strategic partnerships, and rigorous quality practices emerges as a common thread. By leveraging the recommendations outlined herein, stakeholders can navigate complexities, capitalize on new opportunities, and position themselves for sustained success in the dynamic world of lipid-based excipients.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Fatty Acids
- Fatty Alcohols
- Cetyl Alcohol
- Stearyl Alcohol
- Glycerides
- Mixed Glycerides
- Monoglycerides
- Glycerol Monostearate
- Monolaurin
- Triglycerides
- Long Chain Triglycerides (LCTs)
- Medium Chain Triglycerides (MCTs)
- Short Chain Triglycerides (SCTs)
- Phospholipids
- Hydrogenated Phosphatidylcholine
- Soybean Lecithin
- Vegetable Oils
- Coconut Oil
- Sesame Oil
- Waxes
- Beeswax
- Carnauba Wax
- Source of Lipid
- Natural
- Semi-Synthetic
- Synthetic
- Application
- Nasal
- Ophthalmic
- Oral Drug Delivery
- Capsules
- Tablets
- Parenteral
- IV Lipid Emulsions
- Vaccines
- Topical
- Creams
- Patches
- Functionality
- Binders/Fillers
- Emulsifiers & Co-Emulsifiers
- Lubricants
- Penetration Enhancers
- Solubilizing Agents
- Sustained / Controlled Release Agents
- End-User
- Academic & Research Institutes
- Contract Development and Manufacturing Organization (CDMOs)
- Contract Research Organizations (CROs)
- Pharmaceutical & Biopharmaceutical Manufacturers
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- ABITEC Corporation
- American Lecithin Company
- Archer Daniels Midland Company
- Ashland Inc.
- BASF SE
- Cargill, Incorporated
- CordenPharma International GmbH
- Croda International Plc
- Curia Global, Inc.
- Evonik Industries AG
- Gattefossé SAS
- HyCON Labs Solutions.
- IOI Oleo GmbH
- Kewpie Corporation
- Lasenor Emul SL
- Lecico GmbH
- Lipoid GmbH
- Merck KGaA
- NOF CORPORATION
- Pfanstiehl, Inc.
- Polymun Scientific Immunbiologische Forschung GmbH
- Stepan Company
- Stéarinerie Dubois Fils SA
- VAV Life Sciences Pvt. Ltd.
- W. R. Grace & Co.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Lipid-Based Pharma Excipients market report include:- ABITEC Corporation
- American Lecithin Company
- Archer Daniels Midland Company
- Ashland Inc.
- BASF SE
- Cargill, Incorporated
- CordenPharma International GmbH
- Croda International Plc
- Curia Global, Inc.
- Evonik Industries AG
- Gattefossé SAS
- HyCON Labs Solutions.
- IOI Oleo GmbH
- Kewpie Corporation
- Lasenor Emul SL
- Lecico GmbH
- Lipoid GmbH
- Merck KGaA
- NOF CORPORATION
- Pfanstiehl, Inc.
- Polymun Scientific Immunbiologische Forschung GmbH
- Stepan Company
- Stéarinerie Dubois Fils SA
- VAV Life Sciences Pvt. Ltd.
- W. R. Grace & Co.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
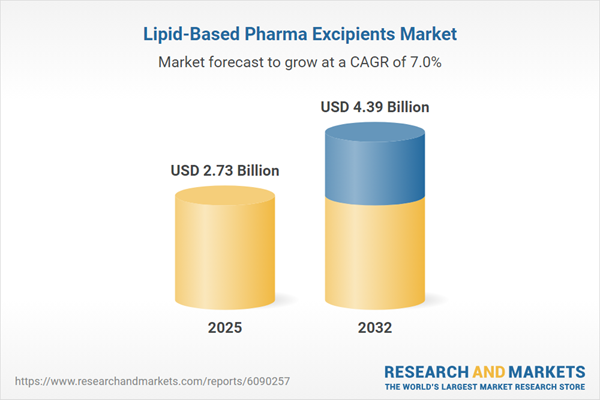
| Estimated Market Value ( USD | $ 2.73 Billion |
| Forecasted Market Value ( USD | $ 4.39 Billion |
| Compound Annual Growth Rate | 6.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |