Speak directly to the analyst to clarify any post sales queries you may have.
Setting the Stage for the Future of Molecular Infectious Disease Testing Amidst Emerging Technological and Regulatory Evolution
The landscape of molecular infectious disease testing has evolved into a critical pillar for global health security and clinical care. As emerging pathogens present new challenges and existing infectious agents develop resistance to therapeutics, diagnostic laboratories and research institutes rely on advanced molecular tools for rapid identification and precise characterization. This convergence of clinical urgency and scientific innovation has elevated the importance of polymerase chain reaction, next-generation sequencing, and other nucleic acid-based techniques in routine workflows.Moreover, regulatory frameworks have grown increasingly rigorous, demanding robust validation and quality management throughout the test development lifecycle. Such oversight, combined with the lessons learned from recent pandemics, has shaped a more resilient and responsive diagnostic ecosystem. Consequently, manufacturers, technology providers, and end users collaborate more closely to accelerate assay approvals and ensure supply chain continuity.
Ultimately, this executive summary provides a concise yet comprehensive foundation for understanding the forces driving molecular infectious disease testing. It lays out transformational shifts, policy impacts, segmentation nuances, and strategic recommendations to equip decision-makers with actionable insights. By synthesizing the latest technological advancements and regulatory imperatives, this introduction sets the stage for a detailed exploration of market dynamics and future directions.
Unveiling Transformative Technological Advances and Market Dynamics Reshaping Molecular Infectious Disease Testing Globally
Rapid innovation in assay design and instrumentation has transformed the molecular testing landscape over the past decade. High-throughput DNA sequencing platforms, once confined to research laboratories, now support routine pathogen surveillance and outbreak investigations. In parallel, advancements in isothermal amplification and microfluidic integration have enabled portable, point-of-care solutions that bring diagnostic capabilities closer to the patient.Furthermore, artificial intelligence and machine learning algorithms are being embedded within software suites to streamline data interpretation, reduce turnaround times, and enhance precision. Cloud-based data management tools facilitate real-time collaboration among diagnostic laboratories, epidemiologists, and public health agencies, fostering a more agile response to infectious threats. Consequently, stakeholders across the value chain are redefining their business models to emphasize service offerings, data analytics, and integrated solutions.
As next-generation technologies converge with decentralized testing approaches, partnerships between established players and emerging innovators have accelerated. Licensing agreements, co-development initiatives, and strategic alliances are driving the rapid deployment of novel assays. Transitional collaborations with academic centers continue to fuel early-stage research, while regulatory agencies refine approval pathways to accommodate breakthrough technologies. This section delves into the transformative dynamics reshaping molecular infectious disease testing and highlights the strategic implications for industry participants.
Assessing the Ramifications of 2025 United States Tariffs on Supply Chains and Pricing in Molecular Infectious Disease Testing
The implementation of United States tariffs in 2025 has introduced new complexities into global supply chains for molecular diagnostics. An array of imported reagents, instruments, and consumables now faces additional duties, elevating cost pressures for manufacturers and end users alike. In response, many suppliers have begun to diversify their sourcing strategies, moving production to tariff-exempt jurisdictions or accelerating domestic manufacturing investments.Consequently, diagnostic laboratories and hospitals have reevaluated procurement contracts, seeking long-term agreements that lock in pricing and secure uninterrupted access to critical materials. This shift has also prompted greater collaboration with local distributors to mitigate potential shortages. In certain cases, organizations have engaged in joint ventures or public-private partnerships to establish regional distribution hubs and buffer inventory against future policy changes.
Ultimately, while the tariff landscape presents cost challenges, it also catalyzes resilience and supply chain innovation. Stakeholders are exploring nearshoring opportunities and strengthening relationships with multiple suppliers to maintain operational continuity. This section examines the multifaceted impact of the 2025 United States tariffs on molecular infectious disease testing, illuminating both the risks and adaptive strategies adopted across the industry.
Understanding Market Segmentation Drivers Across Products Technologies Pathogens Specimens Applications and End Users in Molecular Infectious Disease Testing
A nuanced exploration of product type reveals that high-performance instruments underpin laboratory capabilities, while the recurring revenue streams generated by reagents and kits sustain ongoing diagnostic workflows. Alongside these core assets, software and services have become indispensable for data analysis, quality assurance, and remote monitoring, effectively bridging the gap between raw data generation and actionable clinical insights.Technology segmentation highlights the enduring dominance of polymerase chain reaction in frontline diagnostics, coupled with the rapid adoption of next-generation sequencing for comprehensive pathogen profiling. DNA microarrays continue to offer multiplexed detection, while in situ hybridization provides spatial context in research settings. Meanwhile, isothermal nucleic acid amplification technologies are gaining traction for their simplified workflows and suitability for decentralized testing environments.
From a pathogen perspective, viral targets command substantial attention due to their pandemic potential, yet bacterial and fungal assays remain critical for antimicrobial stewardship. Parasitic infections, particularly in endemic regions, drive specialized testing solutions, while emerging multi-pathogen panels are designed to address co-infection scenarios. Specimen type influences assay development pathways, with blood samples demanding high sensitivity, respiratory and stool specimens requiring robust inhibitor removal, and urine offering noninvasive screening options.
In terms of application, diagnostic testing remains the primary focus, supported by epidemiological investigations that inform outbreak response. Screening testing in high-risk cohorts and therapeutic monitoring for treatment efficacy are increasingly embedded within clinical protocols. End users such as diagnostic laboratories, hospitals and clinics, and research institutes leverage these varied tools to optimize patient outcomes, enhance surveillance programs, and advance scientific discovery.
Highlighting Regional Variations and Growth Catalysts Across the Americas Europe Middle East Africa and Asia Pacific in Molecular Infectious Disease Testing
The Americas region exhibits strong infrastructure for molecular diagnostics, underpinned by substantial investments in laboratory automation and integrated data platforms. North American public health agencies collaborate closely with diagnostic laboratories to implement surveillance networks, while Latin American markets are expanding through capacity-building initiatives and regional partnerships that address emerging infectious threats.Meanwhile, Europe, Middle East and Africa present a mosaic of regulatory requirements and funding models. Western European countries maintain rigorous evaluation pathways for novel assays, fostering high adoption rates for advanced technologies. In contrast, certain markets in the Middle East and Africa prioritize affordability and local manufacturing, leading to tailored solutions and technology transfers that balance cost with performance.
Asia Pacific continues to demonstrate rapid growth driven by escalating healthcare spending and government-led testing programs. Large-scale sequencing initiatives in China and India support pathogen discovery and antimicrobial resistance mapping, while Southeast Asian nations are investing in portable testing platforms to enhance rural healthcare delivery. Across all regions, collaborative frameworks between public institutions and private companies accelerate technology diffusion and strengthen global pandemic preparedness.
Examining Leadership Strategies Innovation Portfolios and Competitive Positioning of Key Players in Molecular Infectious Disease Testing
Leading companies in molecular infectious disease testing have diversified their portfolios through strategic acquisitions and internal innovation. Major diagnostic instrument manufacturers have enhanced their reagent pipelines, while specialized kit developers have expanded into software-driven analytics to deliver end-to-end solutions. In parallel, emerging firms are carving niches with targeted assay panels and rapid point-of-care platforms that complement high-complexity laboratory workflows.Corporate partnerships have also intensified, with alliances linking sequencing technology providers to bioinformatics specialists. Such collaborations facilitate the integration of advanced algorithms for mutation detection, resistance profiling, and phylogenetic analysis. Consequently, customers benefit from consolidated offerings that span sample processing, data interpretation, and result reporting within unified ecosystems.
In addition, downstream service providers are establishing flexible models that combine contract research services with assay development and validation. This trend supports both academic research initiatives and clinical laboratory expansions. As competitive positioning evolves, focus areas include geographic expansion, regulatory approvals in new territories, and the introduction of digital solutions that enhance user experience and data traceability.
Strategic Imperatives for Industry Leaders to Drive Innovation Streamline Operations and Expand Market Footprint in Molecular Infectious Disease Testing
Industry leaders can capitalize on emerging opportunities by first strengthening point-of-care offerings that deliver rapid, decentralized testing in both clinical and outreach settings. Investing in modular platforms allows for seamless integration of new assay chemistries and automated workflows, thereby reducing validation timelines and operational complexity.Furthermore, diversifying supply chains through multi-sourcing agreements and domestic manufacturing partnerships mitigates tariff-related risks. Companies should explore strategic joint ventures with regional distributors to maintain inventory resilience and ensure timely delivery of critical consumables. In parallel, embedding artificial intelligence within software suites enhances data interpretation and predictive analytics, enabling laboratories to detect outbreak patterns earlier and allocate resources more efficiently.
Finally, forging collaborative frameworks with public health agencies, research institutes, and healthcare providers fosters a deeper understanding of real-world needs. Establishing advisory boards and field evaluation programs accelerates market feedback loops and supports regulatory submissions. By aligning product development roadmaps with evolving clinical guidelines, companies will secure leadership positions in the rapidly changing molecular infectious disease testing landscape.
Elucidating the Rigorous Research Methodology Ensuring Data Integrity Analytical Rigor and Comprehensive Coverage of Molecular Infectious Disease Testing
The research underpinning this report combined rigorous secondary analysis of accessible scientific literature, regulatory databases, and publicly available corporate disclosures with primary interviews conducted among a diverse group of industry stakeholders. These interviews included senior executives, laboratory directors, academic researchers, and regulatory experts, ensuring a comprehensive perspective across the value chain.Quantitative data points were validated through triangulation methods, cross-referencing procurement records, supply chain insights, and government agency reports. Qualitative findings were synthesized using thematic analysis to identify recurring trends in technology adoption, competitive strategies, and regulatory developments. Regional nuances were further explored through local market visits and consultations with in-country partners.
This integrated approach ensured that conclusions are grounded in both empirical evidence and the lived experiences of practitioners. By adhering to stringent data integrity protocols and continuously refining analytical frameworks, the report delivers actionable intelligence that supports strategic decision-making for investors, manufacturers, and end users alike.
Synthesizing Core Findings and Implications for Stakeholders Navigating the Evolving Molecular Infectious Disease Testing Landscape
The evolving molecular infectious disease testing landscape reflects a convergence of technological innovation, regulatory adaptation, and geopolitical factors. The emergence of portable diagnostics and advanced sequencing platforms has expanded testing capabilities beyond centralized laboratories, while new tariff structures have prompted supply chain resilience measures. As a result, companies and end users alike must be agile in strategic planning and operational execution.Key findings underscore the importance of segmentation-driven product development, with a balanced emphasis on instruments, reagents, and software services. Regional dynamics further reinforce the need for tailored approaches to address diverse regulatory frameworks and infrastructure maturity levels. In addition, collaborative models spanning public health agencies, research organizations, and private enterprises have proven essential for timely assay validation and deployment.
By synthesizing these insights, stakeholders can better anticipate market shifts, optimize resource allocation, and accelerate innovation. The conclusions presented here offer a foundation for building strategic roadmaps that align with clinical priorities, technological trends, and policy landscapes, ultimately driving more effective responses to current and future infectious disease challenges.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Instruments
- Reagents & Kits
- Software & Services
- Technology
- DNA Microarrays
- DNA Sequencing & Next-Generation Sequencing
- In Situ Hybridization
- Isothermal Nucleic Acid Amplification Technology
- Polymerase Chain Reaction
- Pathogen Type
- Bacterial
- Fungal
- Parasitic
- Viral
- Specimen Type
- Blood
- Respiratory
- Stool
- Urine
- Application
- Diagnostic Testing
- Epidemiological Investigations
- Screening Testing
- Therapeutic Monitoring
- End Users
- Diagnostic Laboratories
- Hospitals & Clinics
- Research Institutes
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Abbott Laboratories
- Agilent Technologies, Inc.
- Altona Diagnostics GmbH
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- BioFire Diagnostics, LLC
- bioMérieux SA
- Cepheid by Danaher Corporation
- Eurofins Viracor LLC
- F. Hoffmann-La Roche Ltd
- GenScript Biotech Corporation
- Grifols S.A.
- Hologic Inc.
- Illumina, Inc.
- Luminex Corporation
- Merck KGaA
- Myriad Genetics, Inc.
- Novacyt Group
- PerkinElmer, Inc.
- Qiagen N.V.
- QuidelOrtho Corporation
- Seegene Inc.
- Siemens Healthineers
- Sysmex Corporation
- Thermo Fisher Scientific, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Molecular Infectious Disease Testing Market report include:- Abbott Laboratories
- Agilent Technologies, Inc.
- Altona Diagnostics GmbH
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- BioFire Diagnostics, LLC
- bioMérieux SA
- Cepheid by Danaher Corporation
- Eurofins Viracor LLC
- F. Hoffmann-La Roche Ltd
- GenScript Biotech Corporation
- Grifols S.A.
- Hologic Inc.
- Illumina, Inc.
- Luminex Corporation
- Merck KGaA
- Myriad Genetics, Inc.
- Novacyt Group
- PerkinElmer, Inc.
- Qiagen N.V.
- QuidelOrtho Corporation
- Seegene Inc.
- Siemens Healthineers
- Sysmex Corporation
- Thermo Fisher Scientific, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
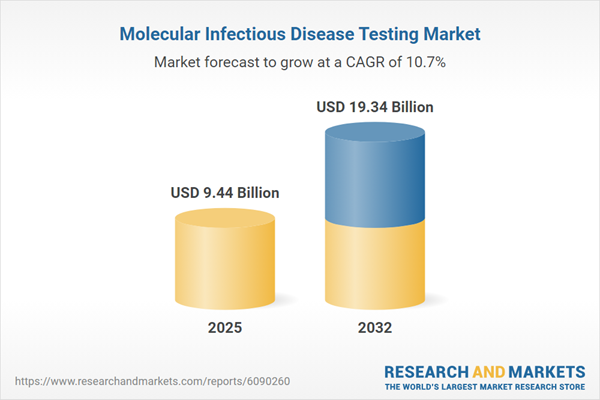
| Estimated Market Value ( USD | $ 9.44 Billion |
| Forecasted Market Value ( USD | $ 19.34 Billion |
| Compound Annual Growth Rate | 10.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |