Speak directly to the analyst to clarify any post sales queries you may have.
Establishing Strategic Foundations in Independent Medical Evaluation Services to Address Growing Demand for Objective Clinical Assessments
Independent medical evaluation services stand at the intersection of clinical precision and legal rigor, providing an essential bridge between healthcare expertise and stakeholder accountability. As the complexity of medical claims intensifies, these evaluations demand unwavering objectivity to inform litigation, insurance adjudication, and employment disputes. Building a robust foundation for this domain requires an appreciation of evolving clinical protocols, stringent regulatory frameworks, and the intricate interplay of multidisciplinary expertise.Against this backdrop, stakeholders increasingly rely on impartial assessments conducted by credentialed professionals who adhere to standardized methodologies. These methodologies draw from evidence-based reporting, ensuring that every opinion is grounded in peer-reviewed data and validated clinical guidelines. Consequently, initial consultations serve as critical entry points, shaping the scope and direction of subsequent expert assessments. The integrity of this sequence underpins the credibility of final reports.
Establishing strategic imperatives begins with aligning service delivery models to emerging trends in telehealth and data analytics. By anchoring operations in transparent processes, service providers enhance trust among corporate employers, insurance carriers, government agencies, and legal firms. As pressure mounts for faster turnaround times and cost-effective solutions, a well-defined introduction to independent medical evaluations becomes indispensable for decision-makers seeking reliable clinical adjudication.
Spotlighting Transformative Shifts Redefining Independent Medical Evaluations Through Telehealth Advancements and Industry-Wide Regulatory Innovation
The landscape of independent medical evaluations is undergoing a profound transformation driven by digital innovation and regulatory evolution. Recent advancements in telehealth platforms have reshaped service delivery, enabling remote assessments that maintain clinical rigor while expanding geographic reach. In parallel, integrated data analytics tools are refining case triage, allowing evaluators to prioritize high-complexity cases and streamline workflows.Regulatory bodies continue to refine guidelines that govern credentialing standards and confidentiality protocols. This regulatory innovation fosters consistency across jurisdictions, ensuring that evaluations adhere to uniform benchmarks for quality and ethical practice. Furthermore, interdisciplinary collaboration between clinicians, legal professionals, and insurance analysts is fostering a cohesive ecosystem in which shared data repositories and secure communication channels enhance transparency.
These shifts underscore the critical need for agility and foresight among service providers. Embracing new technologies while upholding evidence-based reporting standards will determine which organizations emerge as market leaders. As evaluation methods become more sophisticated, the ability to anticipate regulatory changes and leverage digital capabilities will define success in a sector increasingly characterized by rapid adaptation and heightened stakeholder expectations.
Examining the Cumulative Effects of 2025 United States Tariff Policies on Service Delivery Cost Structures and Stakeholder Collaboration Dynamics
The introduction of new tariff policies by the United States in 2025 is poised to create ripple effects throughout the independent medical evaluation ecosystem. These measures, which target medical supplies, diagnostic equipment, and certain pharmaceutical imports, will have a direct bearing on the cost structures of service delivery. As a result, providers must reassess procurement strategies for essential tools and adjust pricing models to preserve margin integrity.Beyond immediate operational expenses, these tariffs carry implications for cross-border collaboration. Evaluators who rely on specialist networks in regions affected by increased duties may encounter delays or higher logistical costs, potentially extending case turnaround times. This scenario necessitates proactive contingency planning, including diversifying supplier bases and exploring domestic sourcing alternatives. At the same time, clients may negotiate new agreement terms to mitigate the impact on budget forecasts and service level commitments.
Ultimately, service providers that anticipate these cumulative effects and recalibrate their strategic roadmaps will safeguard their competitive positioning. By modeling various tariff scenarios and stress-testing financial forecasts, organizations can develop resilient frameworks that maintain service quality while navigating an evolving economic landscape.
Unlocking Key Segmentation Insights Through Multifaceted Analysis of Service Types Evaluation Modes Conditions and End User Profiles for Targeted Strategy Optimization
A nuanced understanding of segmentation insights reveals critical pathways for tailored service innovation. When viewed through the lens of service type, evidence-based reporting remains the cornerstone of many evaluation engagements, while expert assessment enriches clinical nuance and initial consultation streamlines case intake and resource allocation. This layered approach ensures that each phase-from preliminary diagnosis to definitive expert opinion-operates within a coherent framework.Segmentation by evaluation type uncovers distinct operational protocols for physical IME services, which demand on-site examinations and specialized diagnostic tools, versus psychological IME services that emphasize standardized psychometric testing and teleconferencing capabilities. By refining workflows to accommodate these divergent modalities, providers can optimize staffing models and training curricula.
Analyzing delivery modes highlights the growing prominence of remote telemedicine IME services, which complement traditional on-site visits and expand access to specialists across geographies. The integration of virtual platforms enhances scheduling flexibility and reduces overhead, presenting new avenues for scalable service offerings.
Condition type segmentation further underscores the need for specialized knowledge in cardiovascular diseases, neurological disorders, and orthopedic conditions. Tailoring assessment protocols to these clinical categories elevates report accuracy and supports targeted treatment recommendations. Finally, end user segmentation illustrates how corporate employers, government agencies, insurance companies, and legal firms each require bespoke reporting formats and consultative support, underscoring the importance of adaptable service architectures.
Revealing Critical Regional Market Dynamics Shaping Independent Medical Evaluation Services Across Americas Europe Middle East Africa and Asia Pacific
Regional dynamics exert a profound influence on how independent medical evaluation services evolve and gain traction across global markets. In the Americas, the convergence of advanced healthcare infrastructure and stringent insurance regulations drives demand for comprehensive on-site evaluations and rapid turnaround reporting. Providers continually refine logistical frameworks to meet the dual imperatives of accuracy and speed, while aligning with evolving state and federal mandates.Across Europe, the Middle East, and Africa, the landscape is characterized by regulatory heterogeneity and cross-border collaboration initiatives. Service providers leverage regional partnerships and shared data platforms to harmonize evaluation standards, ensuring consistency despite diverse legal frameworks. At the same time, emerging markets within this zone invest in telemedicine capabilities to bridge gaps in specialist availability and reduce dependency on centralized facilities.
In the Asia-Pacific region, a surge in digital health adoption is reshaping service delivery models. Remote IME services powered by sophisticated virtual assessments complement traditional on-site protocols, catering to widespread geographic dispersion and evolving telehealth regulations. As a result, providers are strategically deploying hybrid models that balance technological innovation with deep expertise in region-specific clinical conditions.
Highlighting Leading Companies Driving Innovation Service Excellence and Market Leadership in the Independent Medical Evaluation Sector Through Strategic Initiatives
Leading companies in the independent medical evaluation sector have distinguished themselves through strategic investments in digital infrastructure, specialist networks, and advanced analytics. By integrating cloud-based case management systems with secure communication channels, these organizations expedite report generation while maintaining rigorous data governance and compliance standards.Several prominent service providers have expanded their global footprint by forging alliances with medical centers, legal consultation firms, and insurance carriers. These strategic partnerships enhance access to specialized talent pools and enable rapid scaling of service capacity. Moreover, continuous training initiatives ensure that clinicians remain abreast of evolving clinical guidelines, regulatory updates, and emerging diagnostic technologies.
Innovation at the corporate level also encompasses the deployment of artificial intelligence-driven triage tools that prioritize cases based on urgency and complexity. By automating preliminary data analysis, companies reduce manual workloads and allocate expert resources more efficiently. Collectively, these strategies underscore a competitive landscape defined by agility, collaborative ecosystems, and a relentless focus on service excellence.
Empowering Industry Leaders with Actionable Recommendations to Enhance Efficiency Quality and Competitive Positioning in Independent Medical Evaluation Services
Industry leaders can capitalize on emerging opportunities by adopting a series of targeted recommendations. First, investing in interoperable telehealth platforms will enable seamless integration of virtual and on-site evaluation workflows, enhancing both scalability and patient experience. Providers should prioritize platforms that support secure video consultations, digital signature verification, and robust audit trails to uphold data integrity.Second, diversifying clinical talent pools through cross-training initiatives ensures adaptability to shifting demand across physical and psychological assessment modalities. Comprehensive training programs that encompass condition-specific protocols for cardiovascular diseases, neurological disorders, and orthopedic conditions will strengthen clinical competency and report accuracy.
Third, forging deeper collaborations with key end users such as corporate employers, government agencies, insurance companies, and legal firms will yield bespoke service packages and performance-based pricing models. By co-creating evaluation frameworks, providers can align deliverables with stakeholder objectives, fostering long-term partnerships.
Finally, embedding advanced analytics into case triage and outcome tracking processes empowers providers to identify efficiency bottlenecks and measure impact. Actioning insights derived from real-time dashboards will streamline operations and reinforce continuous improvement mechanisms, driving sustained market leadership.
Outlining Rigorous Research Methodology Combining Qualitative Expert Interviews Quantitative Data Analysis and Comprehensive Literature Reviews
This research employs a rigorous mixed-methods approach that combines qualitative expert interviews with quantitative data analysis. Primary research included in-depth discussions with clinical specialists, legal advisors, and insurance adjudicators to capture diverse perspectives on service delivery challenges and best practices. These interviews informed the development of structured survey instruments designed to validate emerging trends and gauge stakeholder priorities.Secondary research involved comprehensive reviews of peer-reviewed journals, regulatory guidelines, and industry white papers to establish an evidence base for market dynamics and segmentation frameworks. Data triangulation techniques were applied to reconcile insights across multiple sources, ensuring findings reflect a balanced and accurate representation of the independent medical evaluation landscape.
Analytical rigor was further reinforced through statistical modeling to examine correlations between service attributes and performance outcomes. This was supplemented by scenario analyses that stress-test the impact of regulatory shifts and tariff changes on operational resilience. Together, these methodological pillars provide a robust foundation for the strategic recommendations and conclusions presented.
Synthesizing Insights and Strategic Implications to Guide Future Developments in Independent Medical Evaluation Services with Clarity and Precision
The synthesis of segmentation insights, regional dynamics, and competitive benchmarking paints a comprehensive picture of an industry in transition. As service providers navigate rising demands for telehealth-enabled assessments, regulatory harmonization, and evolving cost pressures, strategic agility emerges as the defining characteristic of market leaders. Those who integrate digital innovations with specialized clinical expertise are poised to capture new growth opportunities while safeguarding service quality.Moreover, the cumulative impact of tariff policies underscores the importance of financial resilience and supply chain diversification. Organizations that model various economic scenarios and adapt procurement strategies accordingly will maintain operational stability. Simultaneously, deepening collaborations with end users fosters trust and ensures that evaluation frameworks remain aligned with stakeholder objectives.
Ultimately, the path forward lies in a balanced approach that marries technological advancement, evidence-based protocols, and strategic partnerships. By drawing on the actionable recommendations outlined, industry stakeholders can chart a course toward sustained excellence, driving innovation and delivering reliable clinical adjudication in an increasingly complex environment.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Service Type
- Evidence-Based Reporting
- Expert Assessment
- Initial Consultation
- Evaluation Type
- Physical IME
- Psychological IME
- Mode of Delivery
- On-Site IME Services
- Remote/Telemedicine IME Services
- Condition Type
- Cardiovascular Diseases
- Neurological Disorders
- Orthopedic Conditions
- End Users
- Corporate Employers
- Government Agencies
- Insurance Companies
- Legal Firms
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- California Medical Evaluators
- CBI Health
- CorVel Corporation.
- Direct IME, Corp.
- Enlyte Group, LLC.
- ExamWorks, LLC
- HHC Group.
- IMA Group
- IME Care Center, LLC
- Integrated Medical Evaluations, Inc.
- Leidos, Inc
- MCMC Services, LLC
- Medical Evaluators by MDpanel Company
- MedSource National LLC.
- MES Solutions.
- MLP IME
- Network Medical Review Company, Ltd.
- Saponaro, Inc.
- United Spine and Ortho LLC
- Wisedocs Inc.
- Lex Medicus
- AssessMed
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Independent Medical Evaluation Service Market report include:- California Medical Evaluators
- CBI Health
- CorVel Corporation.
- Direct IME, Corp.
- Enlyte Group, LLC.
- ExamWorks, LLC
- HHC Group.
- IMA Group
- IME Care Center, LLC
- Integrated Medical Evaluations, Inc.
- Leidos, Inc
- MCMC Services, LLC
- Medical Evaluators by MDpanel Company
- MedSource National LLC.
- MES Solutions.
- MLP IME
- Network Medical Review Company, Ltd.
- Saponaro, Inc.
- United Spine and Ortho LLC
- Wisedocs Inc.
- Lex Medicus
- AssessMed
Table Information
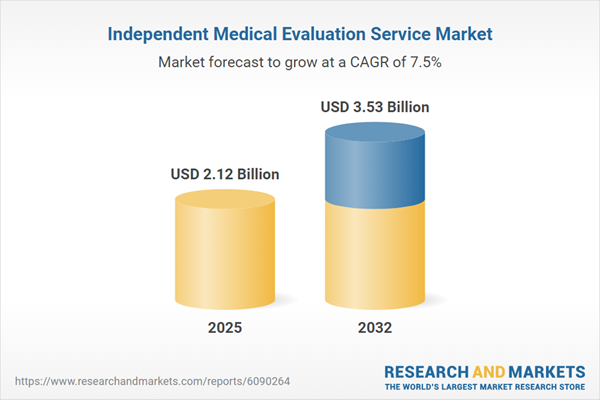
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 2.12 Billion |
| Forecasted Market Value ( USD | $ 3.53 Billion |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |