Speak directly to the analyst to clarify any post sales queries you may have.
The α4β7 integrin antagonists market stands at a transformative point, empowering decision-makers to leverage emerging innovations, differentiated mechanisms, and shifting regulatory contexts to shape commercial outcomes. This report delivers a comprehensive, actionable overview tailored for senior leaders navigating evolving clinical and operational landscapes.
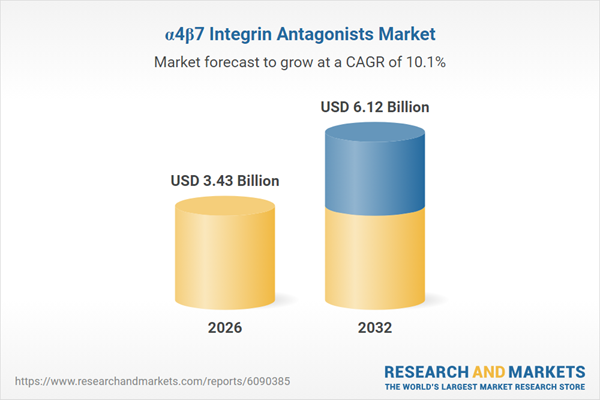
Market Snapshot: Growth Momentum in α4β7 Integrin Antagonists
The α4β7 integrin antagonists market grew from USD 3.12 billion in 2025 to USD 3.43 billion in 2026. Projected at a CAGR of 10.08%, it is forecast to reach USD 6.12 billion by 2032. Robust demand is driven by advancements in antibody engineering, new administration formats enhancing flexibility, and the expanding need for targeted immunology solutions. The therapeutic class continues to attract investment on the strength of its clinical evidence base and regulatory focus in treating chronic inflammatory conditions.
Scope & Segmentation: Critical Market Dimensions
- Drug Type: Examines the roles of Natalizumab and Vedolizumab, each with established regulatory and clinical footprints affecting prescriber and payer dynamics.
- Mode of Administration: Assesses Intravenous and Subcutaneous formats, highlighting differences in patient preference, care setting, and provider economics.
- Mechanism of Action: Includes both Non-Selective Integrin Blockade and Selective β7 Integrin Blockade to clarify implications for safety monitoring and product labeling.
- Therapeutic Application: Covers Crohn's Disease, Inflammatory Bowel Disease, and Ulcerative Colitis—each bringing unique clinical endpoints and payer requirements.
- Regions: Profiles commercial and clinical trends across Americas, Europe Middle East & Africa, and Asia-Pacific, underscoring the variable pace of adoption, differing regulatory nuances, and diverse payer considerations.
- Technological Advancements: Addresses innovations in antibody design, delivery devices, and patient support tools that contribute to administration flexibility and improved adherence.
- Operational Considerations: Reviews supply chain management, risk mitigation strategies for tariffs, and logistics planning spanning sourcing, inventory, and trade policy adaptation.
Key Takeaways: Strategic Insights for Decision-Makers
- Technological advances in antibody engineering and administration formats are reshaping clinical adoption curves by enabling both provider and patient-centric care models.
- Competitive differentiation increasingly depends on selective targeting, comprehensive real-world evidence initiatives, and robust patient support ecosystems integrated into clinical workflows.
- Regional market access strategies must account for local regulatory landscapes, payer negotiation protocols, and distribution models to enable sustainable adoption.
- Strategic partnerships, licensing deals, and supply chain optimization accelerate development timelines and geographical expansion, ensuring resilience amid evolving trade environments.
- Payers and providers are shifting attention toward long-term safety, sustained remission, and patient-reported outcomes, which are influencing formulary inclusion and contracting models.
Tariff Impact: Navigating Trade Policy and Supply Chain Resilience
United States tariff shifts introduce new considerations affecting global sourcing strategies, manufacturing costs, and component procurement for biologic therapeutics. Manufacturers are prioritizing supply chain resilience with diversified sourcing, strategic inventory management, and nearshoring where feasible. Tariffs may alter negotiation dynamics with distributors and payers, requiring integration of trade policy scenarios into procurement and risk planning. These pressures also incentivize process optimization and conditional manufacturing relocation to maintain access continuity for patients.
Methodology & Data Sources
This analysis integrates expert consultation, systematic literature review, and regulatory analysis. Clinical, regulatory, manufacturing, and payer experts provided qualitative inputs cross-referenced against trial registries, regulatory documents, and peer-reviewed studies. Methodological rigor ensured all claims are substantiated and reflect the latest market realities.
Why This Report Matters
- Enables targeted investment and development choices by synthesizing clinical, operational, and commercial levers that affect market access and adoption.
- Equips leaders with early visibility into tariff, supply chain, and regional market shifts, facilitating proactive risk and opportunity management.
- Supports actionable strategy development by aligning evidence generation, administration innovation, and regional execution to payer and provider needs.
Conclusion
Mechanism specificity, delivery innovation, and supply chain strategy converge to define long-term success in the α4β7 integrin antagonists market. Informed, agile planning positions organizations to deliver value and sustain commercial growth as the landscape evolves.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
- Biogen
- C4X Discovery Ltd.
- EA Pharma Co., Ltd.
- Elli Lilly and Company
- Ensho Therapeutics, Inc.
- F. Hoffmann-La Roche AG
- Gilead Sciences, Inc.
- Merck
- Polypharma Group BV
- Protagonist Therapeutics Inc.
- Real-Gene Labs
- RedHill Biopharma Ltd.
- Takeda Pharmaceutical Company Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 3.43 Billion |
| Forecasted Market Value ( USD | $ 6.12 Billion |
| Compound Annual Growth Rate | 10.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |