Speak directly to the analyst to clarify any post sales queries you may have.
Introduction framing the strategic clinical importance and implementation challenges of image-guided navigation across diverse procedural specialties
Image-guided navigation has matured from a niche surgical adjunct into a central pillar of procedural precision, enabling clinicians to navigate complex anatomy with higher confidence and improved intraoperative outcomes. Across specialties, this technology streamlines workflows, reduces reliance on intraoperative fluoroscopy in some settings, and enhances the reproducibility of minimally invasive approaches. As a result, device developers, hospital systems, and third-party service providers are converging around shared priorities: interoperability, procedural efficiency, and meaningful clinical evidence that supports adoption.Clinical adoption is driven by iterative improvements in hardware fidelity, software analytics, and tracking technologies that together reduce cognitive load on surgeons and interventionalists. In parallel, services such as installation, integration, and ongoing education have become differentiators in procurement decisions, reflecting the growing recognition that technology alone does not guarantee better outcomes. Increasingly, procurement teams prioritize solutions that demonstrate end-to-end value through training programs, maintenance models, and seamless integration with existing imaging modalities.
From a regulatory and reimbursement perspective, the pathway to adoption now depends on demonstrable patient and system-level benefits. This requires developers to pair device innovation with robust clinical protocols and real-world performance data. Consequently, strategic planning must balance technological ambition with practical implementation considerations, ensuring that product roadmaps align with the needs of academic centers, high-volume hospitals, and outpatient facilities alike.
How converging imaging and tracking technologies combined with service-centric commercial models are reshaping clinical adoption in surgical navigation
The landscape of image-guided navigation is undergoing transformative shifts driven by technological convergence, new clinical imperatives, and changing delivery models. Imaging subsystems are evolving toward hybrid configurations that blend 2D and 3D capabilities to support different procedural stages. At the same time, tracking modalities are refining their trade-offs: optical tracking continues to deliver high spatial accuracy in open-field environments, while electromagnetic tracking expands applicability within minimally invasive and intraoperative environments where line-of-sight constraints limit optical approaches. These complementary strengths are prompting vendors to offer modular solutions that can be tailored to clinical workflows.Software advances are accelerating capabilities in registration, fusion, and intraoperative visualization, enabling clinicians to synthesize preoperative imaging with live data streams. Machine learning and advanced image processing are beginning to play a role in automating registration steps and highlighting risk zones, which, in turn, reduces setup time and user variability. Concurrently, service models are shifting from transactional sales to outcome-oriented engagement, wherein installation, maintenance, and structured education are bundled with technology to ensure predictable clinical performance.
Regulatory focus on safety and interoperability drives manufacturers to invest in standards-based interfaces and robust verification protocols. In addition, procurement decision-making increasingly weighs total cost of ownership and clinical throughput, causing suppliers to explore new commercial constructs such as managed services, outcome-based contracts, and scalable training platforms. Together, these shifts are redefining competitive advantage and accelerating the integration of navigation systems into routine practice across a wider range of specialties.
Assessment of how new trade measures in 2025 are driving supply chain localization, service resilience, and procurement shifts in navigation device ecosystems
The implementation of tariffs in 2025 has introduced tangible upstream effects on the sourcing, manufacturing, and distribution of image-guided navigation technologies. For companies that rely on globally distributed component supply chains, increased import duties have elevated the cost basis for key hardware elements such as CT scanners, MRI-compatible components, endoscopes, and X-ray fluoroscopy systems. In response, manufacturers are reassessing supplier relationships, consolidating component sourcing where feasible, and accelerating regional manufacturing investments to mitigate exposure to cross-border trade measures.These supply-side adjustments cascade into operational decisions that affect installation timelines and service commitments. When lead times extend due to retooled logistics or the need to qualify alternative suppliers, clinical partners may face delayed integrations and extended training schedules. Consequently, service providers are adapting by investing in local spare-part repositories, cross-training service engineers, and offering phased rollouts that prioritize high-impact features first. This shift toward localized support and inventory reduces downtime risk but can also increase short-term capital allocation for suppliers and providers.
On the demand side, buyers are recalibrating procurement criteria to emphasize total lifecycle resilience. Health systems that manage multiple facilities are prioritizing vendor stability, maintenance responsiveness, and supply chain transparency over the lowest initial purchase price. To maintain access and competitive positioning, vendors are diversifying distribution channels, offering flexible deployment models, and strengthening partnerships with regional distributors. Overall, tariff-induced market friction is forcing a strategic reorientation toward supply chain agility and service robustness, which will likely persist as a core competency for market participants.
Segmentation-driven perspectives showing how component, configuration, technology, clinical application, user type, and distribution choices jointly determine product fit and commercial strategy
Segmentation analysis reveals how component, configuration, tracking technology, application area, end user, and distribution choices create differentiated value propositions across the field of image-guided navigation. When analyzed by component, hardware remains foundational and includes computed tomography scanners, endoscopes, magnetic resonance imaging platforms, positron emission tomography units, single photon emission computed tomography devices, ultrasound systems, and X-ray fluoroscopy equipment; services span installation and integration, maintenance and upgrades, and training and education; software layers enable registration, visualization, and analytics that tie the ecosystem together. This layered structure highlights why partnerships between imaging OEMs, navigation specialists, and clinical training organizations are increasingly common.Configuration segmentation distinguishes between 2D navigation, which supports streamlined workflows for procedures relying on planar imaging, and 3D navigation, which provides volumetric context necessary for complex cranial, spinal, and deep-tissue interventions. Each configuration addresses distinct clinical use cases and workflow constraints, and successful vendors design user experiences that minimize the cognitive load associated with transitioning between 2D and 3D views intraoperatively. Tracking technology choices further shape system capabilities: electromagnetic tracking expands the reach of navigation into environments where line-of-sight is difficult, while optical tracking delivers high-precision guidance in open surgical fields, creating complementary trade-offs that influence procurement decisions.
Application-area segmentation spans cardiovascular interventions, dental surgery, ENT procedures, neurosurgery with brain and spinal navigation subdomains, orthopedic surgery, spinal surgery, and urology procedures, each demanding specific imaging modalities, registration strategies, and ergonomics. End-user segmentation captures the different priorities of academic medical centers and research institutes, ambulatory surgical centers, diagnostic centers, and hospitals, reflecting variation in case complexity, purchasing governance, and budget cycles. Finally, distribution channel segmentation contrasts offline routes such as direct sales and distributor networks with online channels including company websites and third-party portals. Together, these segmentation dimensions explain why no single product or commercial model fits all customers and why configurable, service-rich offerings hold a competitive edge.
Regional overview detailing how Americas, Europe Middle East & Africa, and Asia-Pacific dynamics shape procurement preferences and service expectations for navigation systems
Regional dynamics exert a powerful influence on clinical workflows, reimbursement paradigms, and supplier strategies, with distinct patterns emerging across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, concentrated systems and large hospital networks drive demand for integrated imaging and navigation solutions that scale across multiple facilities. Institutions often prioritize interoperable systems and centralized service agreements to maintain uptime across high-volume centers. This emphasis on integration fosters opportunities for vendors that offer strong installation and maintenance capabilities, as well as flexible financing arrangements suited to large procurement cycles.In Europe, Middle East & Africa, market characteristics vary widely by country and healthcare model. Many regions value modular solutions that can be tailored to constrained capital budgets while meeting rigorous regulatory standards. Cross-border distribution hubs and regional service partners play an outsized role in ensuring timely maintenance and spare-part availability. Regulatory harmonization efforts and regional procurement consortia influence adoption patterns, while high-volume teaching hospitals often act as early adopters for cutting-edge navigation systems.
The Asia-Pacific region demonstrates notable heterogeneity between advanced health systems and emerging markets. High-capacity centers in urban areas pursue general-purpose and specialty navigation solutions to support growing procedural volumes, whereas emerging markets prioritize cost-effective hardware and scalable training programs. Across all regions, a common theme is the increasing importance of local service ecosystems and the need for vendors to balance centralized product development with region-specific deployment and support strategies.
Competitive profile emphasizing how technology integration, clinical partnerships, and service-centric business models determine vendor differentiation and long-term contracts
Competitive dynamics are defined by technology differentiation, clinical partnerships, service ecosystems, and regulatory acumen. Market leaders emphasize integrated offerings that combine high-fidelity hardware with intuitive software and comprehensive service bundles. These firms tend to invest in regulatory pathways and clinical studies that substantiate procedural benefits, which in turn supports adoption among large academic centers and integrated delivery networks. Meanwhile, nimble innovators focus on niche clinical applications or disruptive tracking and visualization improvements, seeking to establish clinical proof points that can be scaled through strategic partnerships or white-label arrangements.Strategic alliances between imaging OEMs, navigation specialists, and systems integrators are common, enabling faster go-to-market execution and richer interoperability. In parallel, companies are differentiating through service models, offering extended warranties, remote diagnostics, and structured education programs that reduce operational risk for buyers. On the product side, portfolios that enable modular upgrades without full-system replacement are gaining traction because they align with hospital capital planning and lifecycle management preferences. Finally, regulatory strategy and post-market surveillance are critical capabilities: organizations that demonstrate robust quality systems and transparent clinical outcomes tend to secure longer-term contracts and repeat engagements with large health systems.
Actionable strategic imperatives for vendors and providers to align product modularity, service excellence, and supply chain resilience with clinical adoption goals
Industry leaders should prioritize a set of strategic initiatives that align product innovation with scalable service delivery and resilient supply chains. First, investing in interoperable architectures and modular hardware platforms will reduce integration friction and extend product lifecycles, enabling customers to adopt incremental upgrades rather than replace entire systems. Second, expanding capabilities in installation, maintenance, and education as packaged services will improve clinical outcomes and reduce perceived risk for buyers, thereby supporting broader adoption across both high-volume hospitals and lower-acuity centers.Third, companies should accelerate regional manufacturing and spare-part localization where tariff exposure or logistics risk is material, while simultaneously strengthening distributor relationships to ensure rapid field support. Fourth, cultivating clinical evidence through collaborative research and real-world data collection will help articulate value in payer and hospital procurement conversations. Fifth, explore outcome-aligned commercial models and flexible financing that reflect total cost of ownership and throughput benefits rather than only upfront capital price. Finally, invest in workforce enablement by developing scalable training curricula and remote support tools that reduce onboarding time and improve reproducibility across diverse procedural teams. Taken together, these actions will enhance competitiveness and reduce the operational friction that currently constrains broader clinical integration.
Comprehensive mixed-methods research approach combining clinician interviews, technical review, and expert validation to ensure credible and actionable insights
The research methodology underpinning this analysis combines structured primary research, comprehensive secondary review, and iterative expert validation to ensure findings are both robust and actionable. Primary research included targeted interviews with clinicians, hospital procurement leaders, service managers, and product specialists to capture firsthand perspectives on clinical workflows, decision drivers, and service expectations. These interviews were purposefully selected across a range of end users, from academic medical centers to ambulatory surgical facilities, to reflect the diversity of adoption contexts.Secondary inputs comprised a systematic review of peer-reviewed clinical literature, regulatory filings, device technical specifications, and publicly available procurement guidelines to contextualize clinical utility, safety considerations, and interoperability requirements. Data synthesis relied on triangulating qualitative insights with documented technical capabilities and policy trends, ensuring that assertions about technology trade-offs and service models reflect both practitioner experience and documented device characteristics. Throughout the process, draft findings were circulated to independent clinical experts and supply-chain specialists for validation and refinement, enabling the identification of practical limitations and opportunities.
Where applicable, methodological limitations are acknowledged: stakeholder perspectives may be weighted by region and specialty representation, and rapidly evolving technology features can change implementation considerations over short time horizons. To mitigate these risks, the approach emphasizes recurring validation and the provision of modular data extracts that support targeted follow-up analyses for specific clinical or commercial questions.
Concluding synthesis highlighting the necessity of integrating technological excellence with resilient support models to scale clinical adoption of navigation solutions
In conclusion, image-guided navigation stands at an inflection point where technological maturity, service innovation, and supply chain strategy jointly determine the trajectory of clinical adoption. The interplay of hardware sophistication, advanced tracking modalities, and software-driven visualization creates meaningful opportunities to improve procedural precision across a wide set of specialties. However, realizing that potential requires vendors to match product advances with robust installation, training, and maintenance services, and to demonstrate clear clinical value that resonates with both clinicians and procurement stakeholders.Market participants face practical challenges from trade policies, supply chain complexity, and regional variation in clinical practice, which in turn elevate the importance of localized support and flexible commercial models. Companies that proactively address these constraints by investing in modular product designs, resilient sourcing, and outcome-oriented service offerings will be best positioned to secure long-term engagements. For institutional buyers, prioritizing interoperability, total cost of ownership, and evidence-based performance will help align procurement decisions with patient safety and operational efficiency objectives.
Overall, the most successful strategies will be those that integrate technological excellence with pragmatic deployment and support capabilities, creating reliable clinical workflows that scale across settings while preserving the capacity for ongoing innovation.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Component
- Hardware
- Computed Tomography (CT) Scanners
- Endoscope
- Magnetic Resonance Imaging (MRI)
- Positron Emission Tomography (PET)
- Single Photon Emission Computed Tomography (SPECT)
- Ultrasound Systems
- X-ray Fluoroscopy
- Services
- Installation & Integration
- Maintenance & Upgrades
- Training & Education
- Software
- Hardware
- Configuration
- 2D Navigation
- 3D Navigation
- Navigation Technologies
- Electromagnetic Tracking
- Optical Tracking
- Application Areas
- Cardiovascular Interventions
- Dental Surgery
- ENT Procedures
- Neurosurgery
- Brain Navigation
- Spinal Navigation
- Orthopedic Surgery
- Spinal Surgery
- Urology Procedures
- End Users
- Academic Medical Centers & Research Institutes
- Ambulatory Surgical Centers
- Diagnostic Centers
- Hospitals
- Distribution Channel
- Offline
- Direct Sale
- Distributor Network
- Online
- Company Websites
- Third-Party Portals
- Offline
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Abbott Laboratories
- Allengers Medical Systems Ltd.
- B. Braun SE
- Brainlab AG
- Canon Medical Systems Corporation
- CASCINATION AG
- ClaroNav
- DB Surgical
- FUJIFILM Holdings Corporation
- GE HealthCare Technologies Inc.
- Happy Reliable Surgeries Pvt. Ltd.
- Joimax GmbH
- Koninklijke Philips N.V
- Medtronic PLC
- Nanjing Perlove Medical Equipment Co., Ltd.
- Olympus Corporation
- OnLume Surgical
- Orthofix Medical Inc.
- RXOOM Healthcare Pvt. Ltd.
- Siemens Healthineers AG
- Smith & Nephew PLC
- Stryker Corporation
- Vista Robotics
- Ziehm Imaging GmbH
- Zimmer Biomet Holdings, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Image-guided Navigation market report include:- Abbott Laboratories
- Allengers Medical Systems Ltd.
- B. Braun SE
- Brainlab AG
- Canon Medical Systems Corporation
- CASCINATION AG
- ClaroNav
- DB Surgical
- FUJIFILM Holdings Corporation
- GE HealthCare Technologies Inc.
- Happy Reliable Surgeries Pvt. Ltd.
- Joimax GmbH
- Koninklijke Philips N.V
- Medtronic PLC
- Nanjing Perlove Medical Equipment Co., Ltd.
- Olympus Corporation
- OnLume Surgical
- Orthofix Medical Inc.
- RXOOM Healthcare Pvt. Ltd.
- Siemens Healthineers AG
- Smith & Nephew PLC
- Stryker Corporation
- Vista Robotics
- Ziehm Imaging GmbH
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
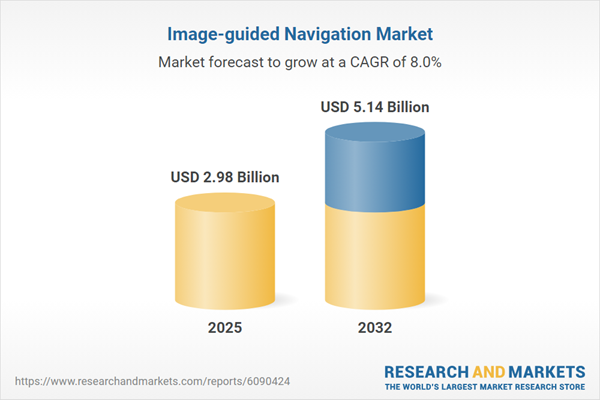
| Estimated Market Value ( USD | $ 2.98 Billion |
| Forecasted Market Value ( USD | $ 5.14 Billion |
| Compound Annual Growth Rate | 8.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |