Speak directly to the analyst to clarify any post sales queries you may have.
Overview of the Technological Foundations and Drivers Shaping the Adoption of Hydrogen Peroxide Vapor Sterilization Equipment Across Critical Industries
MarkdownHydrogen peroxide vapor has emerged as a preferred method for sterilization in environments requiring the highest levels of microbial control. Its ability to achieve rapid, low-temperature sterilization without leaving toxic residues makes it especially valuable for heat- and moisture-sensitive surfaces. This innovative approach leverages advanced chemistry and controlled delivery mechanisms to ensure consistent efficacy across diverse operational settings, from pharmaceutical production suites to biomedical research facilities.
The adoption of hydrogen peroxide vapor sterilization equipment is being propelled by stringent regulatory requirements aimed at minimizing patient and worker exposure to harmful pathogens. As healthcare and life science industries intensify their focus on contamination prevention and facility safety, decision makers are seeking technologies that balance performance with environmental considerations. In addition, the drive toward sustainable practices is elevating the importance of sterilization solutions that reduce water usage and chemical waste, further enhancing the appeal of vapor-phase methods.
This introduction establishes the foundational context for understanding how cutting-edge equipment designs, evolving compliance landscapes, and shifting enterprise priorities converge to define the current market trajectory. By presenting key technological principles and strategic drivers, this section lays the groundwork for deeper analysis in subsequent chapters of the report.
Examining Revolutionary Shifts in Sterilization Practices and Emerging Trends Redefining Hydrogen Peroxide Vapor Applications in Critical Care and Industrial Manufacturing
MarkdownThe landscape of sterilization is undergoing transformative shifts driven by unprecedented demands for automation, connectivity, and data analytics. Leading manufacturers are integrating smart sensor arrays and remote monitoring capabilities into vapor generation systems, which enables real-time process validation and predictive maintenance. These digital enhancements not only streamline operational workflows but also provide comprehensive audit trails that satisfy rigorous compliance audits in highly regulated sectors.
Concurrently, the emergence of flexible, modular equipment designs is facilitating rapid reconfiguration of sterilization lines to accommodate evolving product portfolios and facility layouts. The proliferation of single-use technologies in biopharmaceutical manufacturing has spurred demand for adaptable chambers and generators capable of handling custom cycles without compromising throughput. Moreover, as cross-contamination risks escalate in high-precision industries, the focus is shifting toward containment-centric solutions such as isolators and glove boxes equipped with integrated vapor generators.
These combined innovations are redefining market expectations, creating a competitive environment where agility and scalability are as critical as sterilization performance. The convergence of these trends underscores a new era in which equipment providers must deliver holistic solutions that align with the operational agility and regulatory rigors of modern processing environments.
Assessing the Strategic and Operational Consequences of United States Tariffs Imposed in 2025 on the Hydrogen Peroxide Vapor Sterilization Equipment Supply Chain and Cost Structures
MarkdownThe introduction of tariffs on imported sterilization equipment in 2025 has generated a ripple effect throughout the supply chain, resulting in higher capital expenditures for end users and shifting procurement strategies. Equipment manufacturers sourcing key components from international vendors have been compelled to reprice systems or absorb cost increases, impacting profit margins and altering competitive dynamics. At the same time, distributors and end-user organizations are reevaluating vendor agreements to mitigate financial exposure.
In response, several stakeholders have begun to explore regional manufacturing partnerships and localization strategies to circumvent tariff barriers. This strategic pivot has required accelerated due diligence on alternative suppliers and investment in domestic production capacities. While longer-term cost advantages may emerge from reshoring initiatives, the near-term adjustments in logistics, qualification protocols, and validation processes have placed additional pressure on project timelines and capital planning.
Moreover, the tariff landscape has catalyzed renewed conversations around total cost of ownership, encouraging organizations to adopt lifecycle cost modeling rather than focusing solely on upfront purchase prices. As a result, leasing options, service agreements, and bundled maintenance contracts have grown in prominence, offering more predictable financial structures that help offset the volatility introduced by trade policies. These shifts signal the need for proactive tariff management and strategic supply chain resilience planning in the sterilization equipment sector.
Analyzing Comprehensive Market Segmentation Patterns by Type Product Portability Operation Application and Sales Channels to Illuminate Strategic Opportunities
MarkdownWhen evaluating the market by type, one observes that isolators or glove boxes offer unparalleled containment capabilities for high-risk applications, while sterilization chambers deliver versatile batch processing for medium-scale operations and vapor generators address portable or point-of-use requirements. Each form factor addresses distinct operational priorities, influencing capital investment decisions and deployment strategies.
Investigating product differentiation reveals that integrated systems, encompassing both chamber and generator components in a single turnkey solution, appeal to stakeholders seeking streamlined installation and uniform validation. In contrast, standalone equipment provides modular flexibility that allows end users to augment or retrofit existing infrastructures without comprehensive system overhauls.
Portability considerations highlight fixed systems as the backbone of centralized sterilization facilities, where stable utilities and environmental controls support continuous high-volume processing, whereas portable units cater to decentralized sterilization needs in field hospitals, mobile laboratories, and evolving manufacturing footprints.
Operational mode analysis emphasizes automated systems as a means to achieve reproducible cycles and minimize human intervention, whereas manual systems remain prevalent in smaller facilities or specialized research settings requiring hands-on control. These distinctions inform training programs and maintenance protocols.
Applications span biomedical research, food and beverage processing, medical instrument sterilization, and pharmaceutical production, each demanding tailored cycle parameters for microbial reduction and material compatibility. End-user industries ranging from automotive and aerospace to packaging exhibit unique sterilization requirements driven by component complexity and production throughput.
Finally, sales channel strategies demonstrate that offline direct sales and distributor networks remain essential for delivering personalized service and region-specific support, while online platforms are gaining traction for standardized offerings and rapid procurement of consumables and auxiliary components.
Illuminating Regional Dynamics and Adoption Patterns across Americas Europe Middle East Africa and AsiaPacific Markets for Hydrogen Peroxide Vapor Sterilization Equipment
MarkdownIn the Americas, the demand for hydrogen peroxide vapor sterilization equipment is being propelled by robust growth in biotechnology hubs and pharmaceutical manufacturing clusters. Regulatory agencies in the region have set stringent sterilization standards that favor low-temperature, residue-free methods. This has prompted early adoption of high-throughput chamber systems and integrated generators in leading contract development and manufacturing organizations, driving the need for localized service networks and rapid deployment timelines.
Across Europe, the Middle East, and Africa, diverse regulatory frameworks and varying levels of infrastructure maturity have shaped a segmented adoption landscape. Western Europe's emphasis on environmental sustainability and circular economy principles has accelerated investment in energy-efficient vapor generators, while emerging markets in the Middle East and Africa are prioritizing portable units for field clinics and modular pharmaceutical facilities. Collaboration among regional distribution partners is key to navigating complex import regulations and ensuring consistent after-sales support.
In the Asia-Pacific region, rapid expansion of pharmaceutical and food processing industries, coupled with government incentives for domestic manufacturing, has fueled demand for both automated sterilization chambers and manual glove boxes. Local manufacturers are intensifying R&D efforts to introduce cost-competitive systems adapted to regional power and utility standards. Strategic alliances between global OEMs and regional engineering firms facilitate technology transfer and accelerate time-to-market for advanced vapor sterilization solutions.
Profiling Leading Industry Participants and Their Strategic Initiatives Shaping Competition and Innovation in the Hydrogen Peroxide Vapor Sterilization Equipment Sector
MarkdownLeading manufacturers in the hydrogen peroxide vapor sterilization segment have embarked on a wave of strategic partnerships and technology integrations to maintain competitive advantage. Several global equipment suppliers have invested in proprietary sensor and control algorithms to enhance cycle reproducibility and deliver real-time validation data. These innovations serve as key differentiators when seeking approvals from stringent regulatory bodies in highly regulated industries.
Complementing product development efforts, top-tier companies are forging alliances with service providers and research institutions to extend their solution portfolios. This ecosystem approach enables them to offer comprehensive end-to-end sterilization services, spanning equipment installation, process qualification, staff training, and preventive maintenance. Such holistic offerings resonate with enterprise customers seeking to streamline vendor management and reduce operational complexity.
Another notable trend involves acquisitions of regional distribution enterprises to expand geographic reach and strengthen local support networks. By integrating established sales channels with centralized manufacturing capabilities, leading players can accelerate response times and deliver tailored service agreements that address specific regional requirements. These strategic moves underscore a broader industry shift toward convergence of hardware, software, and service models.
Presenting Actionable Strategic Recommendations for Industry Leaders to Optimize Sterilization Capabilities Enhance Operational Efficiency and Accelerate Market Growth
MarkdownIndustry leaders should prioritize investment in modular, scalable equipment platforms that can adapt to evolving facility layouts and fluctuating throughput demands. By embracing open architecture designs, they can accommodate future upgrades in sensor technology and software capabilities without requiring full system replacements. This approach not only protects capital expenditures but also fosters long-term customer loyalty through continuous performance enhancements.
Strengthening supply chain resilience is equally critical. Executives must forge strategic partnerships with both domestic and international suppliers to diversify component sourcing, mitigate tariff exposure, and reduce lead times. Establishing safety stock agreements and collaborative demand forecasting with key vendors will help stabilize production schedules and prevent costly downtime during market disruptions.
Furthermore, aligning product development roadmaps with emerging regulatory guidelines and sustainability targets will enable organizations to anticipate shifts in compliance and environmental standards. Engaging in active dialogue with regulatory authorities and industry consortia ensures timely adaptation to new requirements, positioning companies as proactive innovators rather than reactive implementers.
Finally, unlocking maximum value from data analytics and remote monitoring features will empower stakeholders to optimize cycle efficiency, predict maintenance needs, and refine standard operating procedures. Embedding artificial intelligence-driven insights into service offerings can create differentiated business models that deliver measurable performance improvements and strengthen competitive positioning.
Detailing Rigorous Research Methodology Integrating Primary and Secondary Sources with Data Validation Processes to Ensure Robust and Reliable Market Insights
MarkdownThis research employs a rigorous methodology that integrates both primary and secondary data sources to ensure comprehensive market coverage. Primary insights were gathered through in-depth interviews with senior executives, process engineers, and validation specialists across pharmaceutical, biotech, and healthcare end users. These interviews provided firsthand perspectives on technological requirements, procurement challenges, and strategic priorities.
Secondary research encompassed a systematic review of public filings, regulatory guidelines, conference proceedings, and peer-reviewed literature. Key data points on equipment specifications, adoption rates, and compliance frameworks were extracted from industry publications and patent databases. This secondary information was cross-referenced with market intelligence platforms to validate emerging trends and competitive benchmarks.
Quantitative data analysis involved triangulating supply chain metrics, import-export statistics, and equipment shipment volumes to identify regional adoption patterns and tariff impacts. The qualitative findings were synthesized into thematic insights, which were then subjected to expert panel review to ensure accuracy and relevance. Throughout the process, data integrity protocols and validation checks were applied to maintain the highest standards of reliability and objectivity.
Synthesizing Key Findings and Critical Takeaways to Provide a Cohesive Understanding of Developments and Future Directions in Hydrogen Peroxide Vapor Sterilization Equipment
MarkdownThe collective analysis underscores hydrogen peroxide vapor sterilization equipment as a critical enabler for high-precision industries seeking reliable, residue-free sterilization solutions. Technological advancements in automation, digital connectivity, and modular design have expanded the potential applications while reducing operational complexity. At the same time, regulatory pressures and sustainability objectives continue to drive adoption of vapor-phase methods over traditional alternatives.
Tariff-induced cost challenges have prompted stakeholders to explore regional manufacturing strategies and total cost of ownership models, ushering in new procurement approaches that balance upfront investments with long-term service agreements. Segmentation insights reveal that tailored solutions-from portable generators for field diagnostics to integrated chambers for large-scale pharmaceutical production-are essential for meeting diverse operational needs. Regional analysis highlights the importance of nuanced go-to-market strategies adapted to regulatory environments and infrastructure maturity.
As competition intensifies, leading firms are expanding their value propositions through strategic alliances, technology integrations, and enhanced service offerings. These efforts will shape the competitive landscape, compelling industry participants to differentiate on performance, flexibility, and data-driven service models. Looking ahead, organizations that embrace agility, collaboration, and continuous innovation will be best positioned to capitalize on emerging opportunities and navigate an increasingly complex global market.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Type
- Isolators or Glove Boxes
- Sterilization Chambers
- Vapor Hydrogen Peroxide (VHP) Generators
- Product Type
- Integrated Systems
- Standalone Equipment
- Portability
- Fixed Systems
- Portable Units
- Operational Mode
- Automated Systems
- Manual Systems
- Application
- Biomedical Research
- Food & Beverage Processing
- Medical Instrument Processing
- Pharmaceutical Production
- End-User Industry
- Automotive & Aerospace
- Biotechnology
- Food & Beverage
- Healthcare
- Packaging
- Sales Channel
- Offline Sales
- Direct Sales
- Distributors
- Online Sales
- Offline Sales
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- 3M Company
- Advanced Sterilization Products (ASP)
- Amira S.r.l.
- Andersen Sterilizers
- CARON Scientific & Services, Inc.
- De Lama SPA
- Ecolab, Inc.
- Fedegari Autoclavi SpA
- Getinge AB
- Labnics Ltd.
- Matachana Group
- Process Solutions Corp.
- Protak Scientific Limited
- Sanqiang Medical Equipment Co Ltd
- Shibuya Corporation
- Shinva Medical Instrument Co., Ltd.
- Steelco SpA
- STERIS PLC
- TEKNOMAR Ltd.
- Tema Sinergie S.p.A.
- TOMI Environmental Solutions Inc.
- Vaisala Oyj
- YOUTH CLEANROOM TECHNOLOGY CO., LTD
- Zhejiang TAILIN Bioengineering
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Hydrogen Peroxide Vapor Sterilization Equipment market report include:- 3M Company
- Advanced Sterilization Products (ASP)
- Amira S.r.l.
- Andersen Sterilizers
- CARON Scientific & Services, Inc.
- De Lama SPA
- Ecolab, Inc.
- Fedegari Autoclavi SpA
- Getinge AB
- Labnics Ltd.
- Matachana Group
- Process Solutions Corp.
- Protak Scientific Limited
- Sanqiang Medical Equipment Co Ltd
- Shibuya Corporation
- Shinva Medical Instrument Co., Ltd.
- Steelco SpA
- STERIS PLC
- TEKNOMAR Ltd.
- Tema Sinergie S.p.A.
- TOMI Environmental Solutions Inc.
- Vaisala Oyj
- YOUTH CLEANROOM TECHNOLOGY CO., LTD
- Zhejiang TAILIN Bioengineering
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | November 2025 |
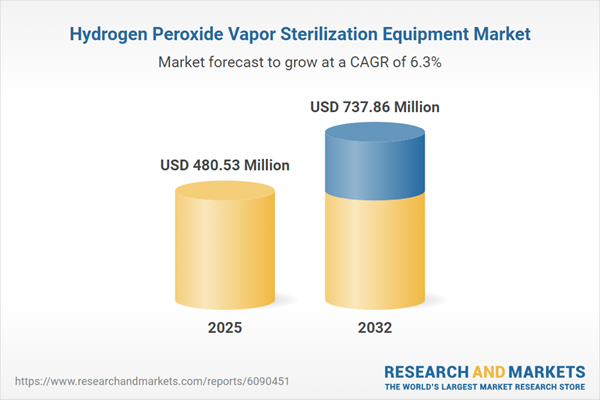
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 480.53 Million |
| Forecasted Market Value ( USD | $ 737.86 Million |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |