Speak directly to the analyst to clarify any post sales queries you may have.
Senior healthcare executives face mounting pressure to ensure infection control, cost optimization, and compliance with evolving protocols. The prophylactic antibiotics market has become a key strategic focus, underpinned by rising surgical volumes and a heightened awareness of antimicrobial stewardship.
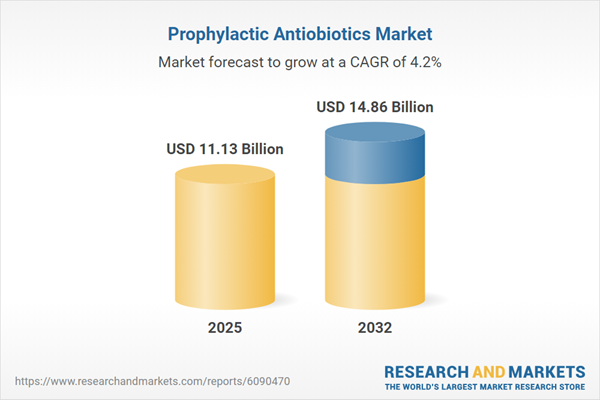
Market Snapshot: Prophylactic Antibiotics
The prophylactic antibiotics market grew from USD 10.72 billion in 2024 to USD 11.13 billion in 2025. It is expected to continue growing at a CAGR of 4.17%, reaching USD 14.86 billion by 2032. This expansion reflects sustained investments in infection prevention, adherence to clinical guidelines, and increased utilization across both developed and emerging healthcare systems.
Scope & Segmentation
The report provides a detailed analysis of the market’s structure, delivering actionable insights for stakeholders by dissecting key segments and regions:
- Type: Cephalosporins, flucloxacillin, gentamicin, tinidazole, vancomycin. Each presents unique clinical profiles and resistance characteristics suited to specific surgical needs.
- Dosage Form: Intravenous, oral, topical, supporting applications ranging from rapid inpatient intervention to convenient outpatient care and localized exposure.
- Application: Dental procedures, gynecological surgery, orthopedic surgery. Each is governed by tailored prophylactic protocols to match varying infection risks.
- End-User: Ambulatory surgical centers, homecare, hospitals and clinics, reflecting diverse administration environments and procurement strategies.
- Distribution Channel: Hospital pharmacy, retail pharmacy, offering distinct pathways for patient access and logistics management.
- Region:
- Americas: United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru
- Europe, Middle East & Africa: United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland, United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel, South Africa, Nigeria, Egypt, Kenya
- Asia-Pacific: China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan
- Companies: Amgen Inc., Biosynth Ltd, GlaxoSmithKline PLC, LEXICARE PHARMA PVT. LTD., Lupin Limited, Merck & Co., Inc., Novartis AG, Novo Nordisk A/S, Pfizer Inc., Roche Holding AG.
Key Takeaways for Senior Decision-Makers
- Antimicrobial stewardship is reshaping prescribing behaviors, aligning infection control with regulatory compliance and patient safety goals.
- Digital health technologies are enabling real-time antibiotic management, increasing adherence and fostering continuous monitoring across diverse care settings.
- Regulatory changes are driving development of innovative formulations and incentivizing collaboration between manufacturers and healthcare providers.
- Regional differences in healthcare infrastructure are leading to varied adoption rates and tailored strategies, particularly in Asia-Pacific and Europe, Middle East & Africa.
- Supply chain resilience is critical, spurring investment in regional manufacturing and adaptive procurement processes to safeguard against disruptions.
Tariff Impact
Recent United States tariff policies have intensified cost challenges throughout the prophylactic antibiotics supply chain. Manufacturers are diversifying supply sources and distributors are revisiting inventory practices in response to these measures. The resulting industry-wide focus on domestic production and public-private collaboration aims to create more stable supply frameworks and reduce vulnerability to external price fluctuations.
Research Methodology & Data Sources
This market analysis leverages a multi-phase approach: review of peer-reviewed scientific literature and regulatory documents, expert interviews with clinicians and procurement leaders, and triangulation of public health, journal, and industry data. Iterative expert panel validation ensures depth and accuracy for all findings.
Why This Report Matters
- Enables executives to benchmark against evolving stewardship initiatives, digital innovations, and supply chain strategies.
- Delivers region-specific and segment-specific nuances, facilitating informed investment, procurement, and policy decisions.
Conclusion
The prophylactic antibiotics market offers actionable opportunities as well as complex regulatory and operational challenges. Leveraging insights from this analysis will empower organizations to optimize outcomes, manage risks, and guide future strategic initiatives.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Prophylactic Antiobiotics market report include:- Amgen Inc.
- Biosynth Ltd
- GlaxoSmithKline PLC
- LEXICARE PHARMA PVT. LTD.
- Lupin Limited
- Merck & Co., Inc.
- Novartis AG
- Novo Nordisk A/S
- Pfizer Inc.
- Roche Holding AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 11.13 Billion |
| Forecasted Market Value ( USD | $ 14.86 Billion |
| Compound Annual Growth Rate | 4.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |