Speak directly to the analyst to clarify any post sales queries you may have.
Charting the Future of Gene Transfer Technologies with Strategic Insights into Emerging Delivery Platforms and Therapeutic Innovations
The field of gene transfer technologies has transcended traditional boundaries to redefine possibilities within modern healthcare. Through the convergence of molecular biology, engineering, and computational tools, researchers and developers are now equipped to manipulate genetic material with unprecedented precision. This transformative ecosystem encompasses both non-viral delivery systems-such as electroporation techniques that use controlled electrical pulses to facilitate gene uptake, lipid-based transfection methods that harness lipid nanoparticles for safe nucleic acid encapsulation, and innovative polymer-based carriers optimized for targeted delivery-and viral vectors that capitalize on naturally evolved mechanisms including adeno-associated viruses with low immunogenic profiles, adenoviruses for robust transgene expression, lentiviruses that enable long-term genetic integration, and retroviruses adept at stable gene insertion.As the global emphasis shifts toward personalized medicine, regenerative therapies, and advanced vaccine platforms, gene transfer has emerged as a foundational pillar. At its core, the discipline is driven by collaborative efforts spanning academic institutes, biopharmaceutical firms, and contract research organizations that collectively shape the trajectory of novel interventions. Consequently, a nuanced understanding of current modalities and future trajectories is essential for stakeholders seeking to harness gene transfer as a catalyst for next-generation treatments and reliable clinical outcomes.
Exploring the Revolutionary Advances and Emerging Trends Driving the Evolution of Gene Transfer Approaches in Modern Biotechnology
In recent years, the landscape of gene transfer has undergone seismic shifts driven by technological breakthroughs and evolving regulatory imperatives. The precision afforded by CRISPR-based genome editing has accelerated research timelines by enabling targeted gene corrections and knockouts at efficiencies once deemed unattainable. Similarly, mRNA delivery systems have demonstrated extraordinary versatility, exemplified by rapid vaccine development during global health crises, thereby underscoring the strategic value of adaptable platforms.Regulatory frameworks have concurrently evolved to address safety, efficacy, and ethical considerations. Accelerated approval pathways have emerged in key markets, enabling expedited clinical evaluations while imposing stringent post-market surveillance. In parallel, digital transformation initiatives have facilitated the integration of artificial intelligence and machine learning algorithms for predictive modeling of vector behavior and patient response profiles. As a result, stakeholders are leveraging real-time data analytics to refine delivery vectors, optimize dosing regimens, and forecast manufacturing demands.
Moreover, collaborative alliances among technology providers, academic centers, and clinical research organizations are fostering open innovation environments. These partnerships are driving the cross-pollination of expertise in bioinformatics, bioprocess engineering, and translational science, further propelling the industry toward a future where gene transfer interventions are safer, more accessible, and tailored to individual patient needs.
Analyzing the Multifaceted Effects of Newly Imposed United States Tariffs on Gene Transfer Supply Chains and Research Dynamics
The implementation of new tariffs in the United States has introduced significant variables into the operational calculus of gene transfer supply chains. Increased duties on critical raw materials, reagents, and specialized equipment have led organizations to reassess supplier relationships and sourcing strategies. Consequently, many firms are exploring alternative procurement channels, including nearshoring and dual sourcing arrangements, to mitigate exposure to trade policy fluctuations and to sustain continuity of experimental workflows.Furthermore, research institutions and contract research organizations are confronting budgetary constraints as cost pressures ripple through grant-funded projects. These developments have prompted innovators to optimize resource allocation by consolidating reagent inventories, enhancing reagent sharing networks, and embracing open-source protocols. In turn, strategic investments in in-house manufacturing capabilities and modular production technologies are gaining prominence as companies seek to insulate critical operations from external pricing volatility.
Despite these challenges, the imperative for groundbreaking therapies remains undiminished. Organizations that proactively adapt their supply chain models, foster collaborative procurement consortia, and engage in policy advocacy stand to preserve momentum in research and development pipelines. Ultimately, the resilience of the gene transfer sector will hinge on its ability to navigate tariff-induced disruptions while continuing to deliver transformative therapeutic solutions.
Unpacking Market Dynamics Through Technology, Application, Therapeutic Area, and End User Perspectives to Reveal Critical Growth Drivers
An in-depth exploration of the gene transfer arena unveils diverse growth vectors shaped by multiple segmentation frameworks. From a technological standpoint, the dichotomy between non-viral and viral delivery approaches highlights opportunities for platform optimization and risk management, with electroporation, lipid-based, and polymer-based systems offering customizable modalities alongside adeno-associated, adenovirus, lentivirus, and retrovirus vectors that leverage evolutionary efficiencies. The array of applications-from gene therapy and personalized medicine initiatives to regenerative treatment strategies and vaccine development programs-further underscores the adaptability of gene transfer platforms across varied clinical objectives.Therapeutic areas open expansive frontiers, encompassing cardiovascular ailments, infectious disease management, metabolic disorder remediation, musculoskeletal repair, neurological condition treatments, and oncology interventions, each demanding bespoke vector properties and delivery considerations. Meanwhile, stakeholders range from academic and research institutions driving fundamental discovery to biopharmaceutical companies scaling production and contract research organizations offering specialized support services. The synergy between these segments forms a dynamic ecosystem where collaborative innovation, targeted R&D focus, and strategic partnerships coalesce to define competitive advantage and accelerate time to clinical impact.
Comparing Regional Opportunities and Challenges Across the Americas, Europe Middle East Africa, and Asia Pacific in Gene Transfer Innovation
Regional distinctions in the gene transfer landscape reflect varying regulatory climates, investment ecosystems, and research infrastructures. In the Americas, robust funding environments and well-established clinical trial networks have catalyzed pioneering applications, particularly in oncology and metabolic disorder gene therapies. Transitioning across the Atlantic and stretching into the Middle East and Africa, regulatory harmonization efforts and public-private partnerships are bolstering capacity for advanced vector production and localized clinical evaluations, while also presenting pathways to address region-specific health challenges.Pivoting further east, the Asia-Pacific corridor exhibits accelerated adoption of gene transfer technologies driven by burgeoning biotech hubs, supportive government policies, and expanding manufacturing footprints. In these markets, partnerships with global technology providers are often complemented by substantial investments in homegrown capabilities, leading to a competitive yet collaborative environment. Collectively, these regional narratives underscore the necessity for tailored market entry strategies, nuanced regulatory navigation, and agile alliances that respond to both local and global imperatives.
Evaluating Leadership Strategies and Competitive Positions of Pioneering Firms Shaping the Gene Transfer Ecosystem Worldwide
Industry frontrunners are distinguishing themselves through differentiated strategies spanning platform refinement, strategic alliances, and expansive service portfolios. Several leading biotechnology firms have prioritized the development of scalable vector manufacturing processes that meet stringent quality standards and accommodate diverse payloads. Simultaneously, specialized contract research organizations are enhancing their service offerings by integrating bioinformatics-driven analytics and automated high-throughput screening capabilities, thereby shortening development cycles and improving experiment reproducibility.Collaboration between academic spin-offs and established biopharmaceutical companies is further shaping the competitive landscape. These strategic partnerships facilitate the translation of early-stage discoveries into scalable clinical solutions, often leveraging co-development arrangements and licensing agreements. Additionally, cross-sector collaborations with digital health innovators and medical device manufacturers are fostering hybrid solutions that combine gene transfer with diagnostic monitoring and targeted delivery systems. Overall, enterprises that blend deep scientific expertise with agile business models are emerging as market catalysts and trendsetters.
Delivering Strategic Recommendations to Propel Leadership and Innovation Amidst Rapid Change in the Gene Transfer Industry Landscape
To navigate the rapidly evolving gene transfer milieu, industry leaders should embrace strategic diversification of delivery platforms by investing in complementary non-viral and viral systems to balance safety, efficiency, and scalability. At the same time, forging ecosystem partnerships with academic centers, contract research organizations, and digital health specialists will amplify innovation pipelines and accelerate translational workflows. In addition, proactive engagement with regulatory authorities can streamline approval pathways through early dialogue and joint scientific advice, while investments in modular manufacturing and localized production hubs will fortify supply chain resilience against external shocks.Moreover, organizations are encouraged to implement data-driven decision frameworks by leveraging real-world evidence, machine learning algorithms, and predictive modeling to refine trial design, optimize vector performance, and anticipate patient response patterns. By enriching their talent pool with interdisciplinary expertise-from gene editing scientists and bioengineers to regulatory affairs specialists and data analysts-companies can bolster their capacity to respond dynamically to emergent opportunities and challenges. Ultimately, a holistic approach that integrates technological innovation, operational agility, and collaborative networks will equip industry leaders to maintain competitive momentum and deliver transformative therapeutic outcomes.
Detailing the Rigorous Research Framework Combining Qualitative Insights and Quantitative Analysis for Comprehensive Market Understanding
This research accomplishment is underpinned by a comprehensive methodology that marries qualitative insights from stakeholder interviews with quantitative analyses derived from primary and secondary sources. To capture the voices of key opinion leaders, dozens of in-depth discussions were conducted with scientists, C-suite executives, regulatory advisors, and manufacturing specialists across multiple geographies. These conversations illuminated real-world challenges and emerging priorities, informing the analytical framework that structures thematic exploration and scenario evaluation.Complementing the primary inputs, secondary research encompassed peer-reviewed journals, patent filings, conference proceedings, and government publications. Data extraction protocols ensured consistency and traceability, while triangulation techniques validated findings against multiple sources. Advanced data modeling tools were employed to synthesize complex variables, explore sensitivity scenarios, and generate actionable insights. Throughout the process, rigorous internal peer review and expert validation rounds safeguarded both accuracy and relevance, culminating in a robust, multidimensional perspective on the gene transfer domain.
Synthesizing Key Findings and Critical Takeaways to Guide Decision Makers in the Expanding Field of Gene Transfer Technologies
The synthesis of these insights underscores the transformative promise of gene transfer technologies to redefine therapeutic paradigms. From the interplay of non-viral and viral delivery innovations to the region-specific ecosystems that enable localized progress, the landscape is characterized by both immense opportunities and intricate challenges. Strategic segmentation highlights how technology preferences, application demands, therapeutic priorities, and end user capacities converge to shape divergent growth pathways.As industry stakeholders grapple with evolving trade policies, regulatory landscapes, and competitive dynamics, a forward-looking stance is paramount. Entities that blend scientific rigor, operational flexibility, and collaborative networks will be best positioned to advance groundbreaking interventions and improve patient outcomes. Ultimately, this executive summary serves as a guiding compass for decision-makers seeking to harness the full potential of gene transfer, informing strategic direction and catalyzing the next wave of innovation in this rapidly advancing domain.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Technology
- Non-Viral Gene Delivery
- Electroporation
- Lipid-Based Transfection
- Polymer-Based Delivery
- Viral Gene Delivery
- Adeno-Associated Virus
- Adenovirus
- Lentivirus
- Retrovirus
- Non-Viral Gene Delivery
- Applications
- Gene Therapy
- Personalized Medicine
- Regenerative Medicine
- Vaccines Development
- Therapeutic Area
- Cardiovascular Diseases
- Infectious Diseases
- Metabolic Disorders
- Musculoskeletal Disorders
- Neurological Disorders
- Oncology (Cancer)
- End User Segments
- Academic & Research Institutes
- Biopharmaceutical Companies
- Contract Research Organizations
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- 4D Molecular Therapeutics, Inc.
- Beam Therapeutics Inc.
- Bio-Rad Laboratories, Inc.
- Bluebird Bio, Inc.
- Cellectis S.A.
- Editas Medicine, Inc.
- F. Hoffmann-La Roche Ltd
- Generation Bio Co.
- Genethon
- Genprex, Inc.
- Horizon Therapeutics plc by Amgen Inc
- Intellia Therapeutics, Inc.
- Lonza Group AG
- MeiraGTx Holdings plc
- Merck KGaA
- Moderna, Inc.
- Novartis AG
- Orchard Therapeutics plc
- Oxford Biomedica plc
- Pfizer Inc.
- Precision BioSciences, Inc.
- Sangamo Therapeutics, Inc.
- Takara Bio Inc.
- Thermo Fisher Scientific Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Gene Transfer Technologies market report include:- 4D Molecular Therapeutics, Inc.
- Beam Therapeutics Inc.
- Bio-Rad Laboratories, Inc.
- Bluebird Bio, Inc.
- Cellectis S.A.
- Editas Medicine, Inc.
- F. Hoffmann-La Roche Ltd
- Generation Bio Co.
- Genethon
- Genprex, Inc.
- Horizon Therapeutics plc by Amgen Inc
- Intellia Therapeutics, Inc.
- Lonza Group AG
- MeiraGTx Holdings plc
- Merck KGaA
- Moderna, Inc.
- Novartis AG
- Orchard Therapeutics plc
- Oxford Biomedica plc
- Pfizer Inc.
- Precision BioSciences, Inc.
- Sangamo Therapeutics, Inc.
- Takara Bio Inc.
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
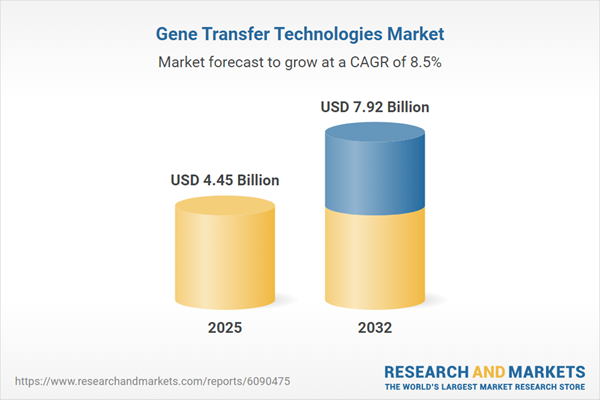
| Estimated Market Value ( USD | $ 4.45 Billion |
| Forecasted Market Value ( USD | $ 7.92 Billion |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |