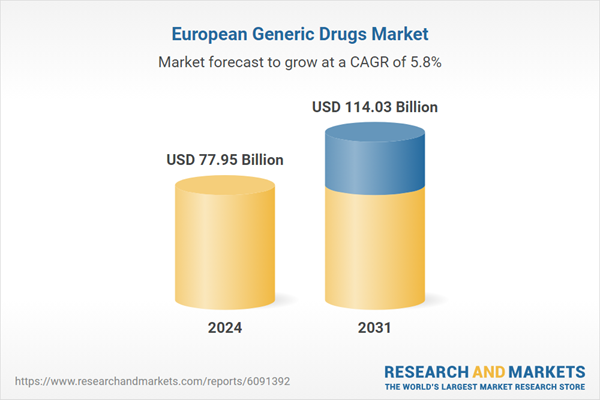
The Europe generic drugs market forecast presented in this report can help stakeholders in this marketplace plan their growth strategies. The Rising Chronic Disease Burden and Increasing Demand for Cost-Effective Treatments and Government Policies and Regulatory Support for Generics are the key factors propelling the Europe generic drugs Market growth.
Rising Chronic Disease Burden and Increasing Demand for Cost-Effective Treatments.
Europe is facing a significant increase in the prevalence of noncommunicable diseases (NCDs). 1 in 6 individuals succumb to these conditions before reaching the age of 70, with cardiovascular diseases, cancer, diabetes, and chronic respiratory illnesses being the primary contributors. As per the World Health Organization (WHO), in July 2024, ~64 million adults and ~300,000 children and adolescents had diabetes in the region, with one in three cases undiagnosed. By 2045, 1 in 10 Europeans may have diabetes, and Europe already carries the highest burden of type 1 diabetes globally. Also, the WHO reported over 4.47 million new cases and ~2 million cancer-related deaths in Europe in 2022.
According to the Organization for Economic Cooperation and Development (OECD) research conducted on OECD member countries in the European Union (EU), such as France, Spain, the Netherlands, Romania, and Portugal, published in February 2025, more than 70% of individuals in these countries living with multiple chronic conditions are prescribed with least three medications, and over one-third take four or more. France and the Czech Republic have the highest percentages of people with two or more chronic conditions, including mental health issues, at 77.24% and 70.13%, respectively.
The surging prevalence of chronic diseases in Europe is driving the healthcare systems and patients in seeking affordable solutions to manage long-term conditions. Generic medications offer cost-effective alternatives to brand-name drugs, making them attractive options for healthcare providers and patients. According to Generics and Biosimilar Initiative, in Europe, generic medicines are generally 20-80% cheaper than brand-name medicines, resulting in significant savings. 67% of dispensed medicine prescriptions are for generics, yet they account for just 29% of total expenditure on medicines. Without competition from generic manufacturers, maintaining this level of access would cost Europe an additional UD$ 113.82 billion (€100 billion) each year.
According to an article published by the European Federation of Pharmaceutical Industries and Associations (EFPIA) in 2025, cancer spending varies from less than US$ 169.75 (€150) per capita in Hungary, Croatia, Romania, Latvia, and Bulgaria to more than US$ 452.68 (€400) in Germany and Switzerland. The high cost of biologics for cancer treatment is contributing to the growing adoption of biosimilars which are relatively cost-effective options available in the market. According to WHO, biosimilars are ~ 60 % cheaper than their reference counterparts. Thus, the rising chronic disease burden is increasing the adoption of cost-effective generic medications for treatment, fueling the market growth.
Factor Hampering Europe generic drugs market
While generics offer a lower-cost alternative to branded medications, the pressure to lower prices to remain competitive forces manufacturers to sell their products at minimal profit. Generic drugs enter the market at a lower price point than branded drugs, often ranging 30-80% less. However, the sheer number of generic manufacturers that tend to enter the market once a patent expires, can lead to price conflicts. As more competitors launch their versions of a generic drug, the prices often drop substantially, thereby compressing profit margins. In 2023, Sandoz reported a 5% drop in operating income from its generics segment, even though revenues grew by 4%.The company highlighted price erosion in Europe, especially for antibiotics and oncology generics, as a major factor impacting its margins. Such impact on profit margins creates challenges for manufacturers to balance producing high-quality drugs while maintaining profitability. The reliance on high-volume sales to sustain profitability means that generic drug manufacturers face substantial risk during market saturation. If too many companies produce the same generic drug, the market becomes flooded with same generic drugs, and prices continue to drop, making it difficult for companies to recover their production and operational costs. In 2021, the UK NHS introduced cost-control measures that reduced reimbursements for generics by 8%.
The Pharmaceutical Price Regulation Scheme (PPRS), which applies to branded and generic drugs, saw its ceiling lowered in 2022, requiring generics manufacturers to provide greater rebates for NHS drugs. As of 2023, the rebate to the NHS from generics producers was estimated to reach nearly US$ 2.64 billion (£2 billion), reflecting the increasing pressure on pricing. This pressure is challenging for small and mid-sized generic pharmaceutical companies, which may struggle to compete with prominent players that have the resources to absorb lower margins. The entry of large pharmaceutical firms into the generic drug space can exacerbate this price competition.
These firms have more resources to lower prices and maintain profitability due to their economies of scale, making it difficult for smaller generic manufacturers to compete. In markets with price pressure, generic drug manufacturers may be forced to prioritize cost-cutting measures in production, compromising on aspects such as quality control or innovation. This prioritization can lead to potential concerns around the safety and efficacy of generics, though regulatory bodies such as the EMA have measures in place to prevent such issues. In conclusion, the intense price competition and low profit margins can create a challenging environment for manufacturers. Companies must navigate these pressures carefully to maintain sustainability and continue to provide affordable alternatives to branded medications.
- Based on product type, the Europe Generic Drugs market is bifurcated into Small Molecule (Fluorouracil (5-FU), Methotrexate, Doxorubicin, Mitomycin, Asparaginase, Carboplatin, Capecitabine, Others) and Biosimilar Products. The Small Molecule segment held a larger share of the Europe Generic Drugs market in 2024
- By drug class, the Europe Generic Drugs market is segmented into Drug Class (Central Nervous System, Cardiovascular, Urology, Rheumatology, Oncology, Hematology and Others). The Others segment held the largest share of the Europe Generic Drugs market in 2024.
- In terms of route of administration, the Europe Generic Drugs market is segmented into Route of Administration (Oral, Injectable, Topical and Others). The Oral segment held the largest share in the Europe Generic Drugs market in 2024.
- By Type, the market is bifurcated into Type (Prescription Drugs and Over-the-Counter Drugs). The Prescription Drugs segment held a larger share of the Europe Generic Drugs market in 2024.
- Based on distribution channel, the market is segmented into Distribution Channel (Hospital Pharmacies, Retail Pharmacies and Online Pharmacies). The Hospital Pharmacies segment held the largest share of the Europe Generic Drugs market in 2024.
Reasons to buy:
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the Europe Generic Drugs Market.
- Highlights key business priorities in order to assist companies to realign their business strategies.
- The key findings and recommendations highlight crucial progressive industry trends in the Europe Generic Drugs Market, thereby allowing players across the value chain to develop effective long-term strategies.
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets.
- Scrutinize in-depth market trends and outlook coupled with the factors driving the Europe Generic Drugs Market, as well as those hindering it.
- Enhance the decision-making process by understanding the strategies that underpin security interest with respect to client products, segmentation, pricing, and distribution.
Table of Contents
Companies Mentioned
Some of the leading companies in the Europe Generic Drugs Market include:- Teva Pharmaceutical Industries
- Viatris Inc
- Dr. Reddy's Laboratories Ltd
- Novartis AG
- Sun Pharmaceutical Industries Ltd
- AbbVie Inc, AstraZeneca Plc
- Sanofi SA
- Aurobindo Pharma Ltd
- Glenmark Pharmaceuticals Ltd
- Hikma Pharmaceuticals Plc
- Cipla Ltd
- GSK Plc
- Eli Lilly and Co
- medac Pharma LLP
- Lupin Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 205 |
| Published | May 2025 |
| Forecast Period | 2024 - 2031 |
| Estimated Market Value ( USD | $ 77.95 Billion |
| Forecasted Market Value ( USD | $ 114.03 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 16 |